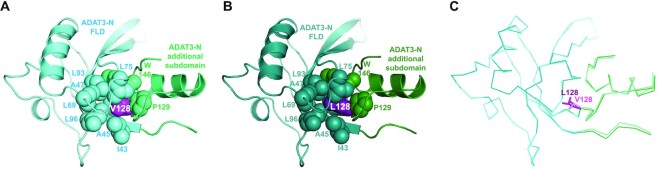
Figure 7.
Position of V128 within ADAT3-N and effect of its mutation in leucine (V128L). (A) Ribbon structure of ADAT3-N with the residues forming its central V128 (purple) hydrophobic core shown as spheres. The residues are coloured according to the subdomain they belong to: FLD (cyan) and additional structural subdomain (aquamarine). (B) Same as (A) for the V128L mutant. The same colour code is used with slightly darker colours. (C) Superposition of the ADAT3-N WT and V128L domains shown as Cα ribbons. Slight changes are observed in the main chain position (movements of 0.3–0.9 Å) of residues from the V128 hydrophobic core and of neighbouring residues upon the V128L mutation. These movements propagate notably within the ADAT3-N specific structural subdomain but much less in the FLD. A V128M mutation is expected to exacerbate these changes.