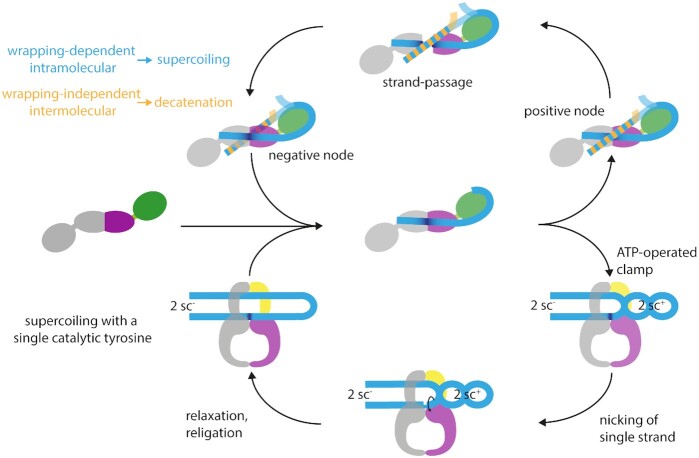
Figure 5.
Possible mechanisms for type IIA topoisomerases. The upper part shows the canonical strand-passage mechanism for DNA supercoiling, depicting gyrase in top view, looking down in the DNA bound at the DNA-gate. The bottom part shows the nicking-closing mechanism, which rationalizes the ability of gyrase to negatively supercoil DNA with only one catalytic tyrosine present (84). Here, the CTDs are not shown as the structural basis for capturing two positive supercoils is unknown. In both mechanisms, the first step is the binding of the G-segment (light blue) to the DNA-gate (one subunit shown in purple, the other in gray). On binding, the G-segment becomes bent (dark blue) at the DNA-gate, and is wrapped around the CTD (green). When ATP binds, the N-gate closes, and the T-segment (blue/orange) is captured. Strand passage (top) can occur with a T-segment located on the same (blue) or a second DNA molecule (orange). Intramolecular strand passage is wrapping-dependent and leads to DNA supercoiling (blue); intermolecular strand passage is wrapping-independent and leads to DNA decatenation (orange). The two catalytic tyrosines cleave the G-segment, whereupon the DNA-gate opens, and the T-segment can pass through the gap, converting a positive into a negative node. Gyrase with a single catalytic tyrosine can supercoil DNA without strand passage, following a nicking-closing mechanism (bottom). Capture of the T-segment leads to the stabilization and segregation of two positive supercoils, resulting in the formation of two negative supercoils in the remainder of the DNA. The ATP-operated clamp inhibits the rotation of the T-segment around its helical axis. Upon induction of a nick in the G-segment by the single catalytic tyrosine, the two positive supercoils can relax, leaving two negative supercoils behind.