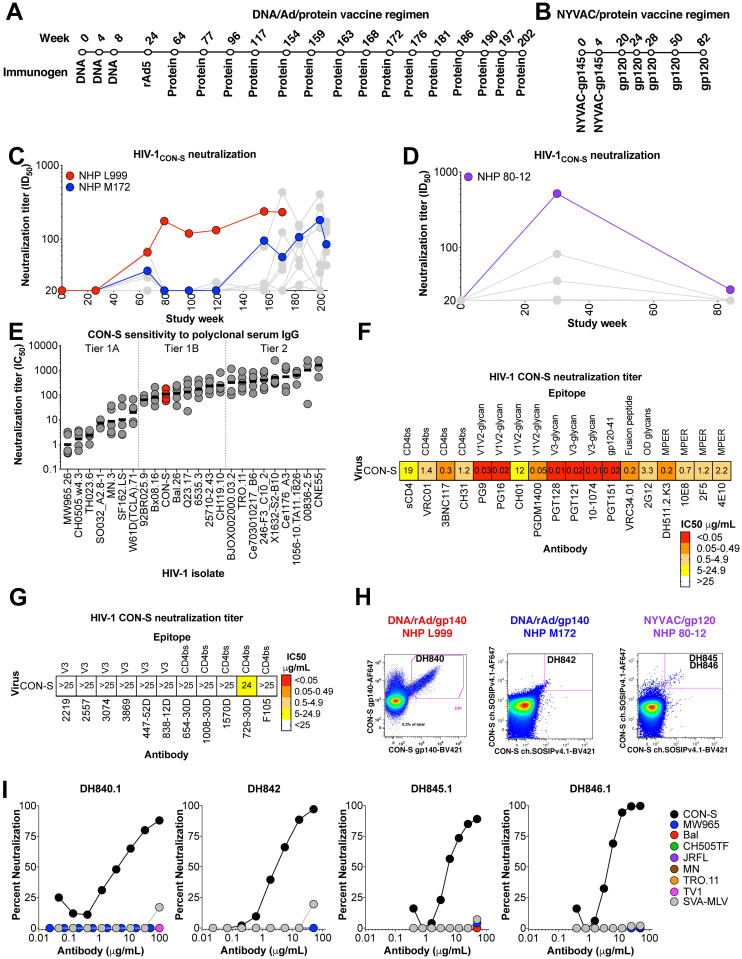
Fig 1. Vaccine elicitation of HIV-1 nAbs in rhesus macaques.
(A) CON-S envelope DNA/recombinant Adenovirus/protein vaccination regimen administered to macaques L999 and M172 (see Materials and methods for details). (B) Macaque 80–12 was immunized with a NYVAC vector expressing gp120 followed by boosting immunization with recombinant gp120. (C,D) Macaque serum neutralization of HIV-1 CON-S infection of TZM-bl cells. Serum or plasma was obtained two weeks after immunization and examined for neutralization. Neutralization titer is shown as reciprocal plasma or serum dilution that inhibits 50% of virus replication (ID50). Macaques from which monoclonal antibodies were isolated are shown in red, blue, and purple. (E) Comparison of neutralization sensitivity of CON-S (red) and other common HIV-1 strains to purified IgG from HIV-1 infected individuals (n = 5). Neutralization titer is shown as IgG concentration in μg/ml that inhibits 50% of virus replication (IC50). Horizontal bars represent the geometric mean of the 5 IgG samples. Vertical dotted lines separate different neutralization tiers. (F,G) HIV-1 CON-S neutralization sensitivity to (F) bnAbs and resistance to (G) linear V3 and poorly-neutralizing CD4 binding site antibodies. Neutralization titer is shown as IC50, and are color-coded based on potency. (H) Antigen-specific single B cell fluorescence-activated cell sorting of the PBMC from each macaque shown in C and D. The recovered antibody of interest is shown within the magenta sort gate. (I) Monoclonal antibody neutralization of HIV-1 infection of TZM-bl cells. Each curve shows the neutralization of different HIV-1 isolates listed in the legend.