Abstract
The prevalence of end-stage kidney disease (ESKD) continuously increases worldwide. The increasing prevalence parallels the growth in the number of people with diabetes, which is the leading cause of ESKD. Early diagnosis of chronic kidney disease (CKD) in patients with diabetes and appropriate intervention is important to delay the progression of kidney function decline and prevent ESKD. Rate of CKD progression and response to treatment varies among patients with diabetes, highlighting the need to tailor individual treatment. In this review, we describe recent advances and areas for future studies with respect to precision medicine in diabetic kidney disease (DKD). DKD is a multi-factorial disease that is subject in part to genetic heritability, but is also influenced by various exogenous mediators, such as environmental or dietary factors. Genetic testing so far has limited utility to facilitate early diagnosis, classify progression or evaluate response to therapy. Various biomarker-based approaches are currently explored to identify patients at high risk of ESKD and to facilitate decision-making for targeted therapy. These studies have led to discovery and validation of a couple of inflammatory proteins such as circulating tumour necrosis factor receptors, which are strong predictors of kidney disease progression. Moreover, risk and drug-response scores based on multiple biomarkers are developed to predict kidney disease progression and long-term drug efficacy. These findings, if implemented in clinical practice, will pave the way to move from a one-size-fits-all to a one-fit-for-everyone approach.
Keywords: chronic kidney disease, diabetic kidney disease, nephropathy, personalized medicine
INTRODUCTION
Precision medicine pursues the idea of tailor-made healthcare decisions, practices or products according to individual characteristics [1]. To achieve this, it is important to accurately diagnose the disease, characterize specific biomarkers that predict disease progression and identify optimal therapy by taking into account individual patient characteristics including the genetic make-up of each patient, lifestyle and environmental factors. Advances in molecular and genetic medicine pave the way for detailed molecular profiling that will help to improve individual pharmacotherapy.
Approximately 700 million people worldwide are diagnosed with chronic kidney disease (CKD) [2]. The most obvious outcome for patients with CKD is kidney failure, but it is well known that cardiovascular disease and premature mortality also occur frequently [3]. Diabetic kidney disease (DKD) is the leading cause of kidney failure, and its classification is based on estimated glomerular filtration rate (eGFR) and albuminuria [4].
The pathophysiology of DKD is multi-factorial, with various pathophysiological factors being involved in the initiation and progression of the disease. Studies have shown that genetic factors contribute to the development of DKD, but these often interact with multiple exogenous factors leading to a weak hereditability pattern [5]. Evidence for a genetic basis of DKD stems from the finding that individuals with familial history of hypertension and cardiovascular disease tend to have higher odds for developing DKD [6]. In addition, siblings of diabetes patients with ESKD have a 5-fold increased risk for developing kidney disease [7]. The prevalence varies among races and ethnic subgroups, suggesting that genetic factors are at least partly involved [8]. Nevertheless, environmental and lifestyle factors are undoubtedly involved. Application of whole-exome sequencing alone may not be particularly useful to guide diagnosis as the true linkage between genetics and disease susceptibility is rather complex. Using genetic approaches for the characterization of the pathophysiology is therefore not as straightforward as in a monogenic disease, where a single gene mutation drives disease progression.
In addition to the heterogeneity in the pathophysiology of DKD, there is also a large heterogeneity in response to treatments. Drugs specifically proven to delay the progression of DKD are angiotensin-converting-enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB). Despite the widespread use of these drugs, prognosis remains poor partly because a substantial proportion of patients do not optimally respond to treatment [9]. These data illustrate that the one-size-fits-all approach in DKD is not applicable to everyone. There is thus a need for individualized treatment approaches focusing on establishing the optimal prognosis and therapy for each individual.
The aim of this review is to provide the current status of precision medicine for patients with DKD. The review will summarize recent precision medicine approaches with respect to the diagnosis, prognosis and response to treatment in these patients.
PRECISION MEDICINE APPROACHES IN MONOGENIC DIABETES
The last decade has seen progress in our understanding of the genetic basis of monogenic causes of diabetes. Neonatal diabetes mellitus (NDM) and maturity-onset diabetes mellitus (MODY) are the most frequently diagnosed forms of monogenic diabetes. The transient NDM is primarily due to overexpression of chromosome 6q24, including the gene PLAGL1 (6q24-q25) and HYMAI (6q24.2). The permanent NDM is caused by activating mutations in the KCNJ11, ABCC8 or the insulin-encoding genes (INS 2004, 2006 and 2007) [10]. Treatment of NDM and MODY using a precision medicine approach has achieved significant progress. For example, it is now known that patients with transient NDM and 6q24 methylation abnormalities respond well to low-dose sulphonyureas derivatives, whereas other NDM require insulin treatment [11]. MODY is an autosomal dominant non-insulin-dependent form of diabetes, where 14 genetic variants have been identified so far, and which is primarily caused by mutations in the HNF1A, HNF4a and GCK genes [12]. Patients with MODY due to GCK polymorphism generally do not respond to either oral anti-hyperglycaemic or insulin therapy [13]. Meanwhile, patients with MODY due to HNF1a or HNF4a mutations are highly sensitive to sulphonyurea derivatives. Dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonist can be further added to optimize glycaemic control [14, 15]. MODY-HNF1B patients respond poorly to any anti-hyperglycaemic drug and thus require insulin as mainstay of treatment [16]. The renal and cardiovascular prognosis of patients with MODY-HNF polymorphism is similar when comparing patients with type 1 and type 2 diabetes [17, 18]. Thus, precision medicine in monogenic causes of diabetes has made significant progress with respect to both risk stratification as well as individualization of therapy.
PRECISION MEDICINE APPROACHES IN DKD
An overview of the various approaches for diabetic kidney disease in the aspect of diagnostic, prognostic, and treatment response prediction are given in Table 1.
Table 1.
Summary of biomarkers implicated in precision medicine model for diabetic kidney disease
| Purpose | Study reference | Biomarkers | Findings and interpretation |
|---|---|---|---|
| Diagnosis | Genetics | ||
| Salem et al. [19] | Col4A3, BMP7, COLEC11 and DDR1 | Among diabetes patients, COL4A3 are associated with glomerular basement membrane thickness. Mutation of COL4A3 leads to heritable nephropathies. BMP7 explicitly expressed in podocytes. Mutations in the COLEC11 and DDR1 gene were associated with impaired collagen synthesis which promote kidney disease progression. | |
| van Zuydam et al. [20] | GABRR1 | GABRR1 is asscociated with the presence of microalbuminuria among European patients with type 2 diabetes. | |
| Pezzolesi et al. [21] | ELMO1 | Polymorphism in the ELMO-1 gene is linked to the development of DKD via up-regulation of type 1 collagen and fibronectin. | |
| Biomarkers | |||
| Ahlqvist et al. [22] | Five clusters based on clinical biomarkers | The clusters-based approach (clusters defined by age, BMI, HbA1c, HOMA-index and the presence of glutamic acid decarboxylase antibodies), successfully reclassified patients with diabetes (type 1/type 2 diabetes), with the goal to optimize diagnosis and prognosis and individualize treatment. | |
| Prognosis | Single proteins | ||
| Niewczas et al. [23] | TNFR1/TNFR2 | TNF receptor predicts eGFR decline and 10-year risk for kidney failure, suggesting that these biomarkers may help in risk stratification. | |
| Coca et al. [24] | KIM-1 | KIM-1 is a predictor of DKD progression in various cohorts indicating that this biomarker can also improve risk stratification. | |
| Protein panels | |||
| Pontillo et al. [25] | CKD273 score | Combined urinary peptide score predicts new onset macroalbuminuria and eGFR decline. | |
| Tofte et al. [26] | CKD273 score | The PRIORITY trial was a prospective trial to validate the prognostic performance of the CKD273 score. The trial did not show that patients with higher CKD273 scores benefited from spironolactone. | |
| Treatment response | Baseline biomarkers for drug response | ||
| Parving et al. [9] | ACE polymorphism | Individuals with DD genotype showed a larger risk reduction for kidney failure during treatment with losartan compared with individuals with the ID or II genotype, suggesting that ACE polymorphisms determine the benefit of ARB treatment. | |
| Idzerda et al. [27] | NT-proBNP | High baseline NT-proBNP was associated with a higher risk of kidney and cardiovascular outcomes but a poorer response to aliskiren. | |
| Dynamic biomarkers for drug response | |||
|
Smink et al. [28] Schievink et al. [29] |
PRE score | The PRE score integrates short-term drug response in clinical parameters (e.g. HbA1c, systolic blood pressure, UACR, body weight, haemoglobin, uric acid and potassium) to predict long-term drug effect on clinical outcomes. The score has been validated for various drugs in patients with type 2 diabetes. | |
BIOMARKER-BASED APPROACHES FOR DIAGNOSIS OF DKD
Genetic biomarkers to improve diagnosis of DKD
Unlike monogenic disease, where genetic testing can accurately confirm a diagnosis, the utility of genetics for establishing a molecular cause of DKD remains elusive. Genome-wide association studies (GWAS) using simultaneous screening of multiple single nucleotide polymorphisms have been conducted to detect susceptibility regions that predispose an individual to DKD, and more than 30 genetic variants for DKD have been identified so far. However, most of the identified genes still need to be confirmed in terms of their functional role in DKD in further replication studies [32]. Recent results from a large GWAS study in patients with DKD revealed that a novel signal near GABRR1 was associated with the presence of microalbuminuria among European subjects. However, no replication of the signal was found among Asian individuals [20]. Another GWAS study conducted in patients with type 1 diabetes with or without kidney disease involving approximately 19 400 participants identified 16 genes associated with DKD. Of these 16 genes, four were spcifically related to glomerular basement membrane collagen and kidney function (COL4A3, BMP7, COLEC11 and DDR1) [19]. These data may help to understand the pathogenesis of DKD in patients with type 1 diabetes. However, it should be mentioned that identification of a genome-wide significant locus with kidney related traits does not necessarily reflect the presence of a causal relationship between a gene and disease susceptibility [33]. For example, a reduction in eGFR among patients with type 2 diabetes was linked to engulfment and cell motility of the ELMO1 gene in chromosome 7p, but variants in UMOD gene located at chromosome 16 were also associated with the progressive loss of kidney function. This indicates that an association between a specific genetic polymorphism and kidney function loss may be a reflection of another causal pathway [21]. Furthermore, the hereditability estimates or proportion of variance explained by a genetic component for the two classical risk markers of DKD are rather low, that is, ∼30–40% for eGFR and ∼4–9% for urinary albumin:creatinine ratio (UACR) [34], supporting possible involvement of other, non-genetic factors.
To overcome some of the shortcomings of using polymorphisms in a single gene, efforts to combine multiple loci with small genetic effects have been initiated to develop a polygenic risk score for DKD [35]. The score is used to estimate an individual's risk for disease over time based upon their genetic liability [36]. However, shared genetic associations identified from GWAS require careful interpretation. Also, incomplete genetic penetrance in DKD and related gene–environment interactions may affect the discriminative ability of the score to guide diagnosis and treatment selection. Hence, more validation studies are needed to fully translate the clinical utility of this score.
Biomarker clusters for improved diagnosis of DKD
Apart from genetic approaches to diagnose and define prognosis of DKD patients, ongoing efforts are underway to improve diagnosis by using clinical characteristics. Based on a data-driven approach, five new clusters of diabetes were introduced according to the absence or presence of glutamic acid decarboxylase, age at diagnosis, baseline level of HbA1c, body mass index (BMI), homeostasis model assessment measured β cell function and insulin resistance. The new cluster approach successfully identified patients with severe insulin resistance with highest risk for DKD development as compared with the other clusters, suggesting the need for early intervention in this group and the possibility to stratify treatment [22].
BIOMARKER-BASED APPROACHES TO IMPROVE PREDICTION OF PROGNOSIS IN HIGH RISK PATIENTS
Early identification of patients at high risk for developing DKD is important for timely initiation of treatment for those at highest risk of adverse outcomes. Highly sensitive and specific prognostic biomarkers will contribute to stratification of patients according to disease progression or estimated overall survival [37].
Single proteins as prognostic biomarkers of DKD
Albuminuria and eGFR are the traditional markers to classify and predict DKD progression in clinical practice and clinical trials [38]. Over the last decade, numerous proteins have been identified from plasma or urine samples that could be potentially added to albuminuria and eGFR. These proteins were commonly linked to single pathological processes in the kidneys such as the inflammation, fibrosis or oxidative stress. For example, 17 proteins from the pro-inflammatory tumour necrosis factor (TNF) superfamily, TNF receptor 1 and TNF receptor 2 (TNFR1 and TNFR2) in particular, were found to be strongly associated with 10-year risk of ESKD [23]. The protein kidney injury molecule-1 (KIM-1) was also identified as one of the highly specific biomarkers to predict DKD progression [24] (Figure 1). Although TNFR1, TNFR2 and KIM-1 have been shown to be strong predictors of DKD progression, it is unclear whether the use of these biomarkers can guide pharmacotherapy to delay kidney function loss. An ongoing study integrates TNFR1, TNFR2 and KIM-1, genetic information and other personal details into an artificial-intelligence-enabled algorithm to predict kidney function decline, aiming to support real-time clinical decision-making and optimize personalized treatment allocation. Initial findings revealed that such clinical and biomarker-driven approaches better predict early kidney function decline as compared with the standard clinical models [39].
FIGURE 1.
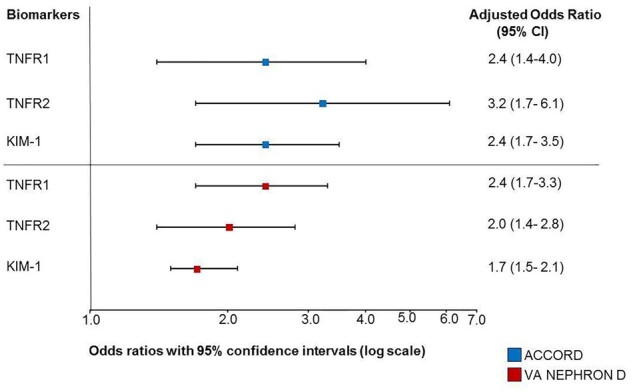
TNFR1, TNFR2 and KIM-1 were strongly associated with renal outcomes in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) and VA NEPHRON-D (Veterans Affairs Nephropathy in Diabetes) trials involving patients with type 2 diabetes at early and advanced stages of CKD [24].
Multiple proteins as prognostic biomarkers of DKD
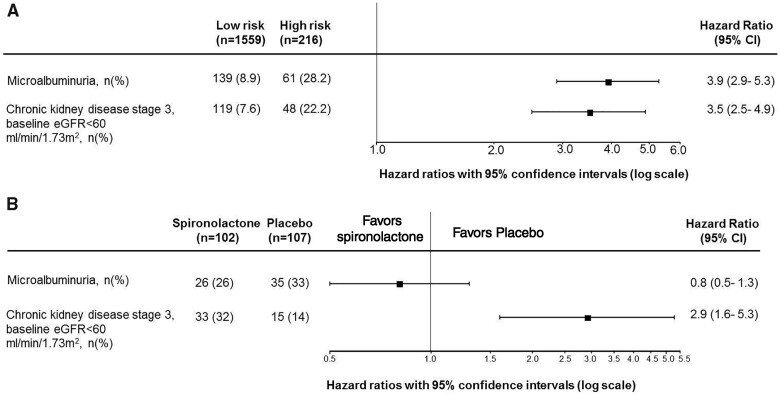
Since DKD is a multi-factorial disease with different pathophysiological processes involved, it is likely that a multiple biomarker panel that captures these processes is better at predicting disease progression than a single biomarker. Development of high-throughput omics profiling enables simultaneous and highly sensitive analysis of various peptides and metabolites in urine and plasma samples. In particular, urinary proteomics has emerged as a promising non-invasive method for diagnostic and prognostic purposes [40]. In an early study among Pima Indians, urinary proteomic profiling has been successfully applied to predict the 10-year risk for development of DKD [41]. Others have developed a urinary proteome-based classifier consisting of 273 peptides (CKD273 classifier) using capillary electrophoresis coupled mass spectrometry. In a prospective case–control study involving 88 patients with type 2 diabetes, the CKD273 classifier predicted the incidence of micro- and macroalbuminuria independently from other clinical parameters [42]. In a large diabetes cohort involving 2087 individuals, the CKD273 classifier accurately predicted eGFR decline and the progression into stage 3 CKD [25]. These initial findings suggest that CKD273 classifier may be used to predict early kidney changes when there is still an option for therapeutic intervention before further structural damage and identify patients in need of early treatment. The PRIORITY (Proteomic prediction and renin angiotensin aldosterone system inhibition prevention of early diabetic nephropathy in type 2 diabetic patients with normoalbuminuria) trial was performed to assess whether spironolactone, a mineralocorticoid receptor antagonist, can be used to reverse nephropathy among high risk patients identified according to the CKD273 classifier [43]. The study showed that, although CKD 273 classifier was beneficial for risk stratification among DKD patients, the risk-based intervention using spironolactone did not reduce the progression of kidney function decline [26] (Figure 2). Further studies with other treatment are needed to clarify the value of CKD273 classifier for optimizing treatment outcomes in DKD.
FIGURE 2.
(A) A higher CKD273 score predicts the risk of microalbuminuria and development of stage 3 CKD. Patients with type 2 diabetes and normoalbuminuria were classified with the CKD273 score into a high risk or low risk group. Patients stratified into the high risk group showed a higher risk of developing microalbuminuria and CKD stage 3 during follow-up [26]. (B) High risk patients with type 2 diabetes and normoalbuminuria based on the CKD273 classifier in the PRIORITY trial were also randomly assigned to treatment with spironolactone or placebo. Spironolactone did not reduce the risk of development of microalbuminuria (primary endpoint). Among participants with baseline eGFR >60 mL/min/1.73 m2, spironolactone increased the risk of stage 3 CKD [26].
BIOMARKER-BASED APPROACHES TO IMPROVE DRUG RESPONSE PREDICTION
Biomarkers can also be used to stratify patients based on their expected response to treatments. They can be measured before a patient is exposed to an intervention. Based on the biomarker level, a decision can be made as to whether therapy is indicated. For this approach, patients are exposed for a short time to the intervention (e.g. a few weeks), and biomarker concentrations are measured before and after the intervention. The short-term changes in the biomarkers are then used to predict the long-term efficacy of the drug. In this approach, the biomarker is referred as a dynamic biomarker, as opposed to baseline biomarkers that are directly associated with the outcome [44].
Baseline biomarkers
Predictive biomarkers can be genes or proteins measured in blood or urine. An example of a predictive genetic biomarker is the ACE polymorphism that modifies the response to renin–angiotensin–aldosterone system (RAAS) inhibitors. A pharmacogenetic study from the RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan) trial has demonstrated that an insertion (I)/deletion (D) polymorphism influences the response to the ARB losartan among DKD patients. Specifically, patients with DD genotype showed marked reductions in kidney failure when receiving losartan as compared with those having the ID or II genotype [9]. Also, in a study among CKD patients without type 2 diabetes, those having the DD genotype had greater treatment response to the ACEi ramipril than those with the ID or II genotype [45]. A genetic polymorphism in the angiotensin II type-1 receptor (AT1R) A1166C was proposed to predict the blood pressure response to ARB, but most studies failed to confirm this hypothesis [46].
An example of a potential plasma protein biomarker that could predict response to RAAS intervention is N-terminal B-type natriuretic peptide (NT-proBNP). It is known that type 2 diabetes patients with higher levels of NT-proBNP tend to have higher cardio-renal risk. Most of the intervention studies then aim to reduce the occurrence of primary cardiovascular event among this high risk group by intensifying treatment. However, identifying these patients does not mean that they will optimally respond to an intensified treatment. A post hoc analysis of the Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints (ALTITUDE) trial showed that patients with the highest NT-proBNP levels at baseline were at highest risk of cardiovascular complications, but did not respond to the investigational drug aliskiren. Thus, selecting patients based on high baseline NT-proBNP for treatment does not necessarily mean that these patients will respond, tolerate or benefit most [27]. Moreover, the novel cluster-based diagnosis of diabetes did not outperform simple model based on patient characteristics for individual treatment selection [47].
Dynamic biomarkers
An optimal dynamic biomarker that predicts the efficacy or safety of a drug is the one that reflects the mechanisms of action of the drug and is ideally involved in DKD progression. An example of a biomarker to reflect the efficacy and safety of an anti-hypertensive drug is blood pressure. However, most drugs have multiple effects on cardio-renal risk markers. For example, sodium–glucose co-transporter 2 inhibitors (SGLT2i) decrease not only HbA1c, but also body weight, blood pressure and albuminuria [48]. Changes in each of these risk markers may contribute to the long-term benefit of these drugs. Additionally, individual patients show different responses to these risk markers. For example, HbA1c may not decrease during treatment with an SGLT2i, yet there may be a profound blood pressure and albuminuria reduction [49]. This suggests that changes in multiple biomarkers should be used to predict the long-term drug effect. Another example is atrasentan, an endothelin receptor antagonist (ERA) that reduces albuminuria. ERAs cause sodium retention in some patients that may lead to oedema and heart failure. Interestingly, at an individual patient level, the albuminuria-lowering response does not correlate with the body weight response, suggesting that some patients may benefit from ERA while not experiencing fluid retention [50]. Thus, balancing the pharmacologic actions of this drug would equate to maximizing the albuminuria response while minimizing sodium retention. The SONAR (Study of Diabetic Nephropathy with Atrasentan) trial was designed according to this concept [51]. Eligible patients started a 6-week open label run-in ‘enrichment’ period during which they received atrasentan 0.75 mg/day. Responder patients, defined as a reduction in UACR of more than 30% from baseline at the end of enrichment who tolerated the drug, with body weight change no more than 3 kg or a B-type natriuretic peptide level, no more than 300 pg/ml, continued in the trial and were randomly assigned to continue atrasentan or placebo. Patients who did not tolerate the drug were not randomized into the double-blind treatment phase. In addition, 1020 patients who tolerated atrasentan but had a UACR reduction less than 30% were also randomized in a separate stratum to assess the effect of atrasentan in these so-called non-responders.
The results of the trial showed that atrasentan significantly reduced the risk of kidney failure by 35% in responders. Interestingly, there was also a tendency towards clinical benefit in the non-responders group, with a 25% relative risk reduction. The explanation of a benefit in non-responders is unknown, but it is possible that atrasentan showed a legacy effect in non-responders or that the large within-individual variability in UACR hampers the separation of responders and non-responders. Alternatively, it is also possible that atrasentan exerts kidney protective effects through pathways unrelated to UACR.
Given this complexity, it seems appropriate to develop a score that integrates the early changes in multiple cardiovascular and renal risk markers of a drug to predict its long-term clinical effect. Such a score, a multiple parameter response efficacy (PRE) score, has been developed. Studies with ARB [28], glucagon-like peptide receptor agonists (GLP1-RA) [29], ERA [30] and SGLT2i [31] have shown that integrating short-term changes in multiple biomarkers into a score perform better in predicting the long-term effect of a drug than changes in any single biomarker (Figure 3). At the individual level, the PRE score also predicted who will benefit from an ARB. Further validation studies are needed before this score can be implemented in daily practice.
FIGURE 3.
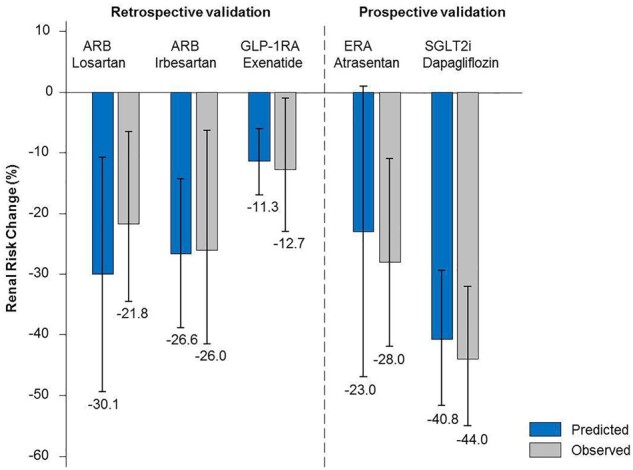
Performance of a multiple PRE score in predicting the effect of various drugs on kidney outcomes using the short-term change in multiple biomarkers. The performance of the score was assessed in completed clinical trials with known outcomes (retrospective validation), and using data from phase 2 studies to predict the outcomes of ongoing phase 3 studies (prospective validation). The score was applied to trials with ARB, losartan (RENAAL trial) and irbesartan [IDNT (Irbesartan Diabetic Nephropathy Trial) trial]; the Glucagon like Petide-1 Receptor Agonists exenetide [EXSCEL (Exenatide Study of Cardiovascular Event Lowering) trial]; the endothelin receptor antagonist atrasentan (prediction of the SONAR trial) and the SGLT2i dapagliflozin [prediction of the DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) trial]. The predicted effect of the drug is shown in blue bars and the observed effect in grey bars.
CONCLUSION
Advances in genetics and molecular biology have provided new avenues for biomarker discovery and personalized medicine for patients with DKD. Significant progress has been made in diagnosis and disease progression prediction, but individualizing the optimal medication for the right patient at the right time remains a challenge. A genetic-based approach to unravel the pathophysiology and find genetic markers for diagnosis and prognosis is often insufficient, as many other exogenous factors are involved in DKD progression. Advances in omics technology have fuelled discovery of novel protein-based biomarkers that may contribute in risk stratification and treatment selection. These biomarkers typically reflect a single mechanism. The use of a single biomarker is unlikely to fully capture the underlying complex and heterogeneous pathophysiology of DKD and treatment responses. Multiple biomarker-based approaches, such as the PRE score, have shown potential to predict long-term drug effects in DKD. The advantage of such scores for individual patients has to be shown prospectively in order to move from a ‘one-size-fits-all’ to a ‘one-fit-for-everyone’ approach.
CONFLICT OF INTEREST STATEMENT
S.C.T. and P.D. report no conflicts of interest. H.J.L.H. is a member of the steering committees of the SONAR and DAPA-CKD trials. He is consultant to Abbvie, AstraZeneca, Boehringer Ingelheim, Janssen, Gilead, Fresenius, Merck, Mundipharma and Mitshubishi Tanabe.
FUNDING
This article is part of a supplement supported by a sponsorship from Amicus Therapeutics UK Limited, a research grant from Boehringer Ingelheim RCV GmbH & Co KG, an educational sponsorship agreement from Astellas Pharma, and a restricted research grant from Vifor Pharma Österreich GmbH. This supplement is part of the project DC-ren that has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 848011.
Contributor Information
Sok Cin Tye, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Petra Denig, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Hiddo J L Heerspink, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
REFERENCES
- 1.National Research Council (US) Committee on A Framework for Developing a New Taxonomy of Disease. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington, DC: National Academies Press, 2011 [PubMed] [Google Scholar]
- 2. Bikbov B, Purcell C, Levey AS et al. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 381: 339. [DOI] [PubMed] [Google Scholar]
- 4. Matsushita K, van der Velde M, Astor BC et al. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kato M, Natarajan R. Diabetic nephropathy–emerging epigenetic mechanisms. Nat Rev Nephrol 2014; 10: 517–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harjutsalo V, Groop PH. Epidemiology and risk factors for diabetic kidney disease. Adv Chronic Kidney Dis 2014; 21: 260–266 [DOI] [PubMed] [Google Scholar]
- 7. Bleyer AJ, Sedor JR, Freedman BI et al. Risk factors for development and progression of diabetic kidney disease and treatment patterns among diabetic siblings of patients with diabetic kidney disease. Am J Kidney Dis 2008; 51: 29–37 [DOI] [PubMed] [Google Scholar]
- 8. Davis TM, Coleman RL, Holman RR et al. ; UKPDS Group. Ethnicity and long-term vascular outcomes in type 2 diabetes: a prospective observational study (UKPDS 83). Diabetes Med 2014; 31: 200–207 [DOI] [PubMed] [Google Scholar]
- 9. Parving HH, de Zeeuw D, Cooper ME et al. ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol 2008; 19: 771–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barbetti FM, Liu M, Grasso V et al. Neonatal diabetes: permanent neonatal diabetes and transient neonatal diabetes. Diabetes associated with single gene defects and chromosomal abnormalities. In: Barbetti F, Ghizzoni L, Guaraldi F (eds). Front Diabetes. Karger: Basel, 2017, 1–25
- 11. Lemelman MB, Letourneau L, Greeley SAW. Neonatal diabetes mellitus: an update on diagnosis and management. Clin Perinatol 2018; 45: 41–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johns Hopkins University. 606391: Maturity-Onset Diabetes of the Young; MODY. Online Mendelian Inheritance in Man. https://www.omim.org/entry/606391
- 13. Chakera AJ, Steele AM, Gloyn AL et al. Recognition and management of individuals with hyperglycemia because of a heterozygous glucokinase mutation. Diabete Cares 2015; 38: 1383–1392 [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 2020; 43: S14–S31 [DOI] [PubMed] [Google Scholar]
- 15. Delvecchio M, Pastore C, Giordano P. Treatment options for MODY patients: a systematic review of literature. Diabetes Ther 2020; 11: 1667–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaxillaire M, Froguel P. Monogenic diabetes in the young, pharmacogenetics and relevance to multifactorial forms of type 2 diabetes. Endocr Rev 2008; 29: 254–264 [DOI] [PubMed] [Google Scholar]
- 17. Dubois-Laforgue D, Cornu E, Saint-Martin C et al. Diabetes, associated clinical spectrum, long-term prognosis, and genotype/phenotype correlations in 201 adult patients with hepatocyte nuclear factor 1B (HNF1B) molecular defects. Diabetes Care 2017; 40: 1436–1443 [DOI] [PubMed] [Google Scholar]
- 18. Hattersley AT, Greeley SAW, Polak M et al. ISPAD Clinical Practice Consensus Guidelines 2018: the diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes 2018; 19: 47–63 [DOI] [PubMed] [Google Scholar]
- 19. Salem RM, Todd JN, Sandholm N et al. ; SUMMIT Consortium, DCCT/EDIC Research Group, GENIE Consortium. Genome-wide association study of diabetic kidney disease highlights biology involved in glomerular basement membrane collagen. J Am Soc Nephrol 2019; 30: 2000–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Zuydam NR, Ahlqvist E, Sandholm N et al. ; Finnish Diabetic Nephropathy Study (FinnDiane). A genome-wide association study of diabetic kidney disease in subjects with type 2 diabetes. Diabetes 2018; 67: 1414–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pezzolesi MG, Katavetin P, Kure M et al. Confirmation of genetic associations at ELMO1 in the GoKinD collection supports its role as a susceptibility gene in diabetic nephropathy. Diabetes 2009; 58: 2698–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahlqvist E, Storm P, Karajamaki A et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018; 6: 361–369 [DOI] [PubMed] [Google Scholar]
- 23. Niewczas MA, Pavkov ME, Skupien J et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 2019; 25: 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coca SG, Nadkarni GN, Huang Y et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 2017; 28: 2786–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pontillo C, Zhang ZY, Schanstra JP et al. Prediction of chronic kidney disease stage 3 by CKD273, a urinary proteomic biomarker. Kidney Int Rep 2017; 2: 1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tofte N, Lindhardt M, Adamova K et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol 2020; 8: 301–312 [DOI] [PubMed] [Google Scholar]
- 27. Idzerda NMA, Persson F, Pena MJ et al. N-terminal pro-brain natriuretic peptide (NT-proBNP) predicts the cardio-renal response to aliskiren in patients with type 2 diabetes at high renal and cardiovascular risk. Diabetes Obes Metab 2018; 20: 2899–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smink PA, Miao Y, Eijkemans MJ et al. The importance of short-term off-target effects in estimating the long-term renal and cardiovascular protection of angiotensin receptor blockers. Clin Pharmacol Ther 2014; 95: 208–215 [DOI] [PubMed] [Google Scholar]
- 29. Schievink B, de Zeeuw D, Smink PA et al. Prediction of the effect of atrasentan on renal and heart failure outcomes based on short-term changes in multiple risk markers. Eur J Prev Cardiolog 2016; 23: 758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Idzerda NMA, Clegg LE, Hernandez AF et al. Prediction and validation of exenatide risk marker effects on progression of renal disease: insights from EXSCEL. Diabetes Obes Metab 2020; 22: 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idzerda NMA, Stefansson BV, Pena MJ et al. Prediction of the effect of dapagliflozin on kidney and heart failure outcomes based on short-term changes in multiple risk markers. Nephrol Dial Transplant 2020; 35: 1570–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gu HF. Genetic and epigenetic studies in diabetic kidney disease. Front Genet 2019; 10: 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tam V, Patel N, Turcotte M et al. Benefits and limitations of genome-wide association studies. Nat Rev Genet 2019; 20: 467–484 [DOI] [PubMed] [Google Scholar]
- 34. Tin A, Kottgen A. Genome-wide association studies of CKD and related traits. Clin J Am Soc Nephrol 2020; 15: 1643–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wuttke M, Li Y, Li M et al. ; V. A. Million Veteran Program. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 2019; 51: 957–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khera AV, Chaffin M, Aragam KG et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018; 50: 1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naesens M, Anglicheau D. precision transplant medicine: biomarkers to the rescue. J Am Soc Nephrol 2018; 29: 24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heerspink HJL, Greene T, Tighiouart H et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 2019; 7: 128–139 [DOI] [PubMed] [Google Scholar]
- 39. Nadkarni GN, Fleming F, McCullough JR et al. Prediction of rapid kidney function decline using machine learning combining blood biomarkers and electronic health record data. bioRxiv 2019; doi:10.1101/587774; pre-print, not peer reviewed
- 40. Zurbig P, Siwy J, Mischak H. Emerging urine-based proteomic biomarkers as valuable tools in the management of chronic kidney disease. Expert Rev Mol Diagn 2019; 19: 853–856 [DOI] [PubMed] [Google Scholar]
- 41. Otu HH, Can H, Spentzos D et al. Prediction of diabetic nephropathy using urine proteomic profiling 10 years prior to development of nephropathy. Diabetes Care 2007; 30: 638–643 [DOI] [PubMed] [Google Scholar]
- 42. Roscioni SS, de Zeeuw D, Hellemons ME et al. A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia 2013; 56: 259–267 [DOI] [PubMed] [Google Scholar]
- 43. Siwy J, Schanstra JP, Argiles A et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transpl 2014; 29: 1563–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sechidis K, Papangelou K, Metcalfe PD et al. Distinguishing prognostic and predictive biomarkers: an information theoretic approach. Bioinformatics 2018; 34: 4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perna A, Ruggenenti P, Testa A et al. ; for the Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). ACE genotype and ACE inhibitors induced renoprotection in chronic proteinuric nephropathies. Kidney Int 2000; 57: 274–281 [DOI] [PubMed] [Google Scholar]
- 46. Schelleman H, Stricker BH, Verschuren WM et al. Interactions between five candidate genes and antihypertensive drug therapy on blood pressure. Pharmacogenom J 2006; 6: 22–26 [DOI] [PubMed] [Google Scholar]
- 47. Dennis JM, Shields BM, Henley WE et al. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol 2019; 7: 442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heerspink HJ, Perkins BA, Fitchett DH et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus. Circulation 2016; 134: 752–772 [DOI] [PubMed] [Google Scholar]
- 49. Petrykiv SI, Laverman GD, de Zeeuw D, Heerspink HJL. The albuminuria-lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes Metab 2017; 19: 1363–1370 [DOI] [PubMed] [Google Scholar]
- 50. de Zeeuw D, Coll B, Andress D et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with Type 2 diabetic nephropathy. J Am Soc Nephrol 2014; 25: 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heerspink HJL, Parving H-H, Andress DL et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 2019; 393: 1937. [DOI] [PubMed] [Google Scholar]