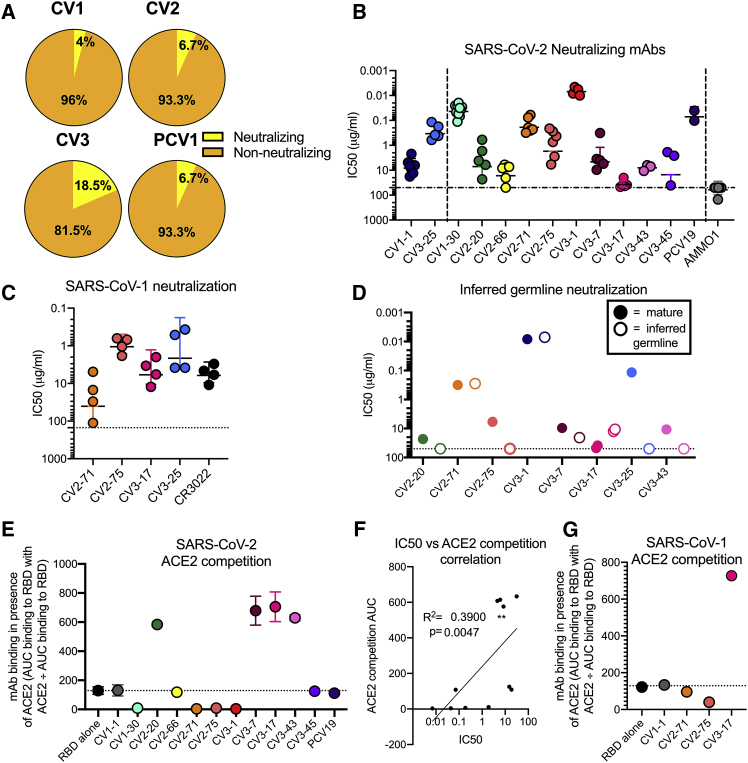
Figure 4.
SARS-CoV-1 and SARS-COV-2 cross-neutralizing properties of mAbs
The 14 neutralizing mAbs were characterized.
(A) Percentage of mAbs capable of achieving 50% neutralization of SARS-CoV-2 pseudovirus at a concentration of 50 μg/ml from each donor.
(B) The IC50s of each nAb in comparison to a negative control (AMMO1) are graphed. Each data point represents an independent replicate, with the mean and SD indicated with error bars. The non-RBD-binding mAbs, CV1-1 and CV3-25, on the left side of the graph are separated by a dashed line from the RBD-binding mAbs on the right side of graph.
(C) SARS-CoV-2 neutralizing mAbs were assessed for their ability to neutralize SARS-CoV-1. CR3022 is a control SARS-CoV-1 neutralizing mAb. Horizontal line indicates mean with error bars at SD. Full data in Figures S4A–S4D.
(D) The IC50s of the iGL versions of the mAbs (open dots) are compared to IC50s of mutated mAbs (solid dots). Additional data in Figure S5.
(E) Competition of mAbs for binding to ACE2. mAbs that show competition have a binding signal below the dotted line and block ACE2 binding, and mAbs with a binding signal above the dotted line enhance ACE2 binding by increasing avidity through immune complex formation. Competition is calculated as the area under the curve (AUC) of mAb binding to the RBD in the presence of ACE2 divided by the AUC of mAb binding to RBD alone. Dots are shown as the median of two replicates, with SD indicated by error bars. The dotted line at the RBD-alone condition indicates BLI signal of uninhibited RBD:ACE2 binding. The NTD-specific CV1-1 mAb is used a negative control.
(F) Correlation between SARS-CoV-2 neutralization IC50 with AUC of the BLI of competition with ACE2 for RBD binding. R2 value for nonlinear fit and Spearmen correlation p value are shown.
(G) The competition of mAbs for binding to SARS-CoV-1 RBD with ACE2 is compared on this graph performed as in (E). Full ACE2 competition data in Figures S4F–S4J. Additional characterization of CV1-1 and CV2-75 in Figure S6.