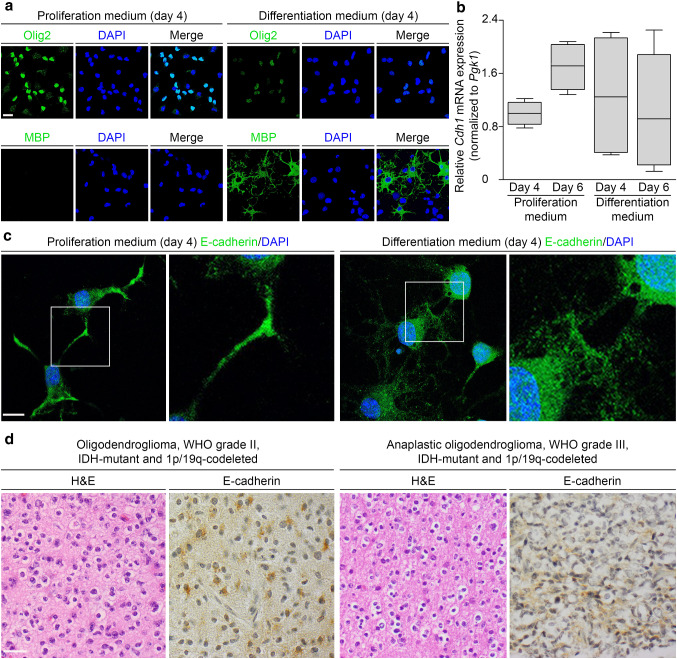
Fig. 2.
CDH1 is expressed in rat primary oligodendroglial cultures and in some human ODs, WHO grade II/III. a–c Cdh1 expression of primary oligodendroglial cultures isolated from neonatal Sprague–Dawley rats (P0–P3) and cultivated for four or six days in proliferation or differentiation medium was analyzed on the RNA and protein level. Prior to expression analysis, differentiation status of oligodendroglial cells was determined by immunostaining of oligodendrocyte transcription factor 2 (Olig2) and myelin basic protein (MBP). After incubation in proliferation medium, most cells were Olig2-positive while MBP was not detected, consistent with oligodendrocyte precursor cells; after incubation in differentiation medium, a substantial population of MBP-positive cells was observed, indicating differentiated oligodendrocytes; scale bar 15 µm (a). Cdh1 mRNA was measured using SYBR green-based real-time RT-PCR and normalized to Pgk1 mRNA. Appreciable levels of Cdh1 mRNA expression were detected irrespective of medium and culture duration with the highest levels in oligodendroglial cultures after six days of proliferation medium. Expression data from four independent experiments performed in triplicate are presented as box plots (b). By immunofluorescence, E-cadherin expression in oligodendroglial cultures was localized to the cytoplasm and slightly enhanced at cellular extensions and regions of cell–cell contacts irrespective of culture medium used; scale bar 10 µm (c). By immunohistochemistry, E-cadherin was expressed in three of 19 (15.8%) human CDH1 wildtype ODs, WHO grade II/III. Depicted are two E-cadherin-positive ODs, WHO grade II/III and consecutive hematoxylin–eosin (H&E) stained sections; scale bar 30 µm (d)