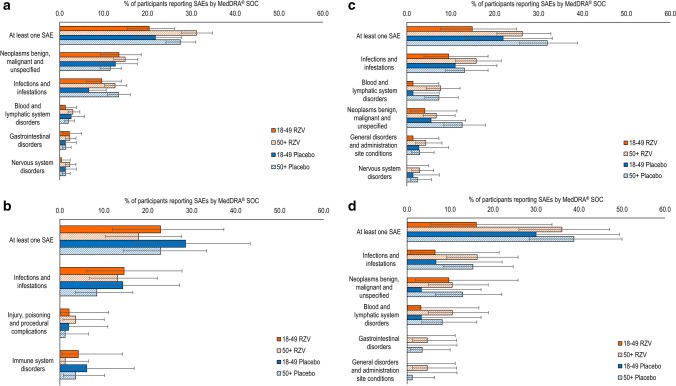
Fig. 4.
Serious adverse events (SAEs) by age. a Adjuvanted recombinant zoster vaccine RZV and placebo in autologous hematopoietic cell transplant recipients [phase III] (Bastidas et al. [42]); b RZV and placebo in renal transplant recipients (Vink et al. [45]); c RZV and placebo in patients with hematologic malignancies (Dagnew et al. [43]); and d RZV and placebo in patients with solid organ tumors (Vink et al. [46]). Figure panels present the percentage of RZV recipients reporting at least one unsolicited adverse event categorized under that Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (SOC). Only the five SOCs with the greatest percentage of RZV participants reporting any event are presented here. Because of the comparatively small sample sizes in the RZV groups in the studies with autologous hematopoietic stem cell transplant recipients (phase I/II) and human immunodeficiency virus-infected adults, we were not able to generate meaningful data for age strata in these studies