Fig. 5.
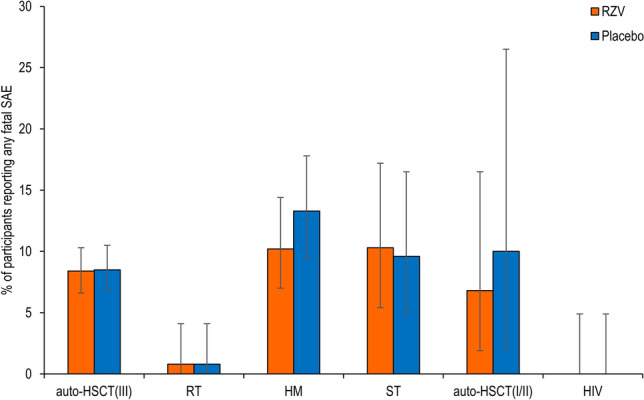
Fatal serious adverse events (SAEs). auto-HSCT(I/II) autologous hematopoietic stem cell transplant recipients (phase I/II), auto-HSCT(III) autologous hematopoietic stem cell transplant recipients (phase III), HM hematologic malignancies patients, HIV human immunodeficiency virus-infected adults, RT renal transplant recipients, RZV adjuvanted recombinant zoster vaccine, ST solid tumors patients. The auto-HSCT(I/II) study includes data for both groups receiving two and three doses of RZV, respectively
