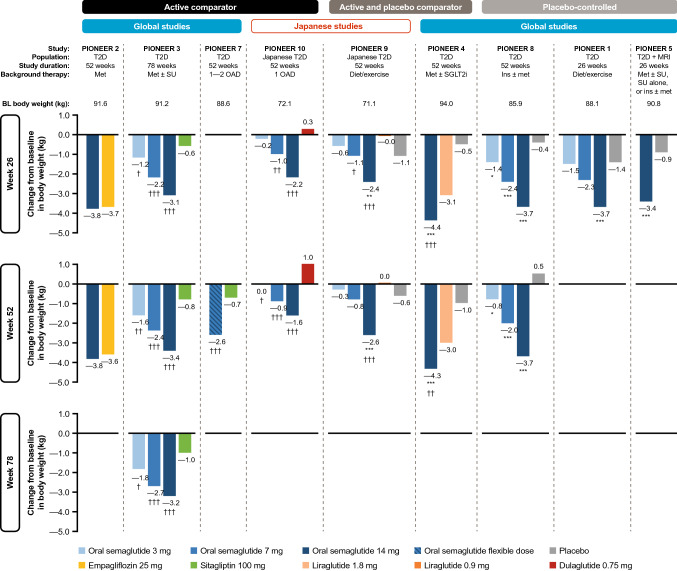
Fig. 3.
Reductions in body weight with oral semaglutide in the PIONEER program. *p < 0.05, **p < 0.01, ***p < 0.001 favoring oral semaglutide vs. placebo. †p < 0.05, ††p < 0.01, †††p < 0.001 favoring oral semaglutide vs. active comparator. §p < 0.05, §§p < 0.01, §§§p < 0.001 favoring active comparator vs. oral semaglutide. Data are for the treatment policy estimand (regardless of premature treatment discontinuation or rescue medication use). BL baseline, ins insulin, met metformin, MRI moderate renal impairment, OAD oral antidiabetes drug, SGLT2i sodium-glucose co-transporter-2 inhibitor, SU sulfonylurea, T2D type 2 diabetes