Figure 4. Role of HIF-1 under normoxia, hypoxia and pseudohypoxia.
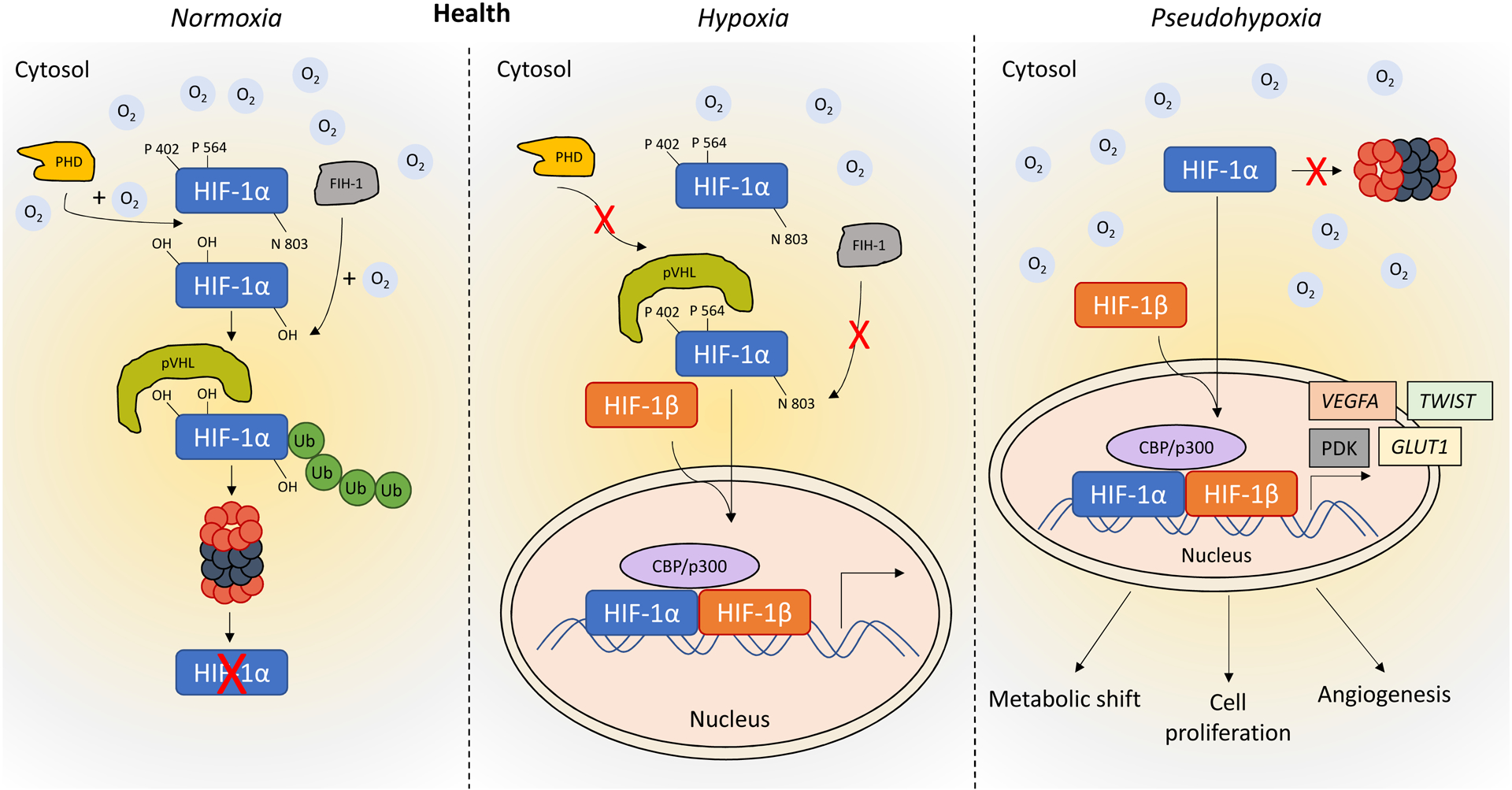
In normoxic conditions, prolyl hydroxylases (PHDs) modify proline residues within the HIF-1α subunit. This allows the von Hippel-Lindau protein (VHL) to ubiquitinate HIF-1α, targeting it for proteasomal degradation. Under hypoxic conditions, PHD is inactivate. As a consequence, HIF-α is not targeted for degradation by VHL. This allows HIF-α to accumulate within the cytoplasm and translocate into the cell nucleus where it dimerizes with HIF-1β to form the active HIF complex. HIF complexes in the nucleus are capable of binding to hypoxia response elements (HRE) located within regulatory elements of genes known to control processes related to oxygen delivery and oxygen deprivation, including pathways involved in cell metabolism, angiogenesis, erythropoiesis, cell proliferation, and apoptosis. Normoxic activation of HIF-1α (pseudohypoxia, as seen in PAH and cancer) contributes to the metabolic shift, cell proliferation and angiogenesis.
