Figure 1. Recruitment of neutrophils by a tumor.
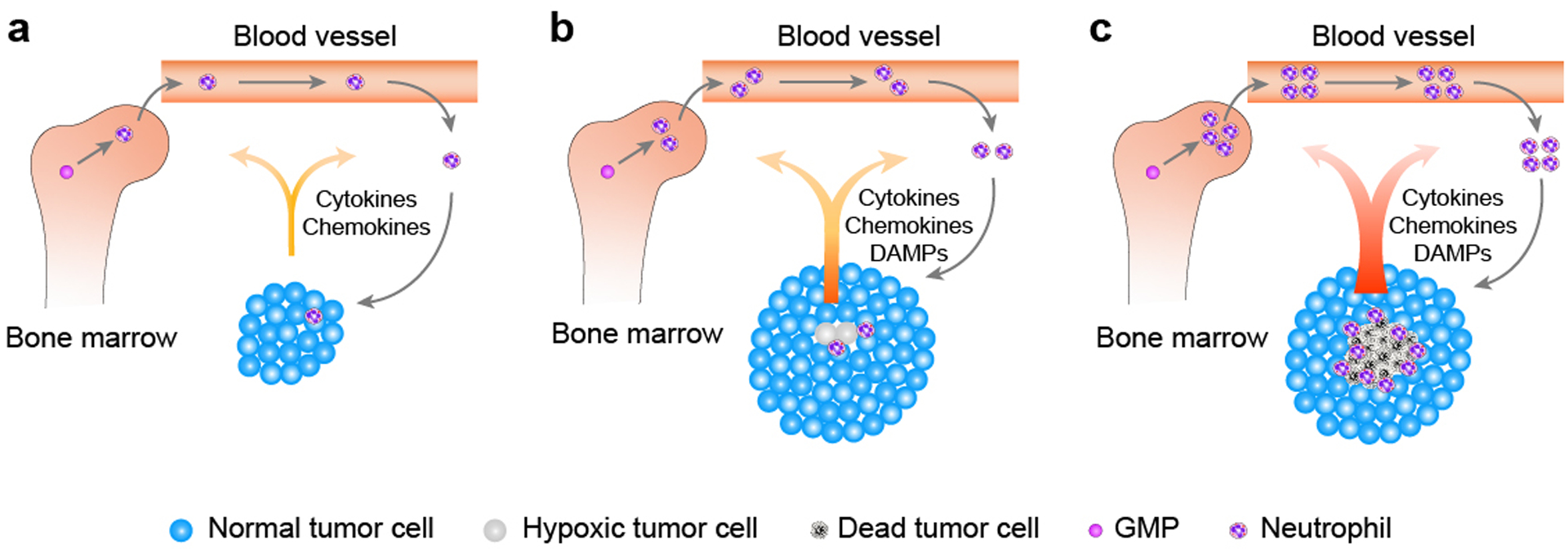
a) Under normal physiology, neutrophils arise from granulocyte-monocyte progenitors (GMPs) in bone marrow and are released upon maturation, and the whole process is tightly regulated. In the context of cancer, both production and retention of neutrophils are disrupted. Tumor cells overproduce a variety of granulopoiesis-promoting factors, which not only pathologically promote granulopoiesis in the bone marrow but also overpower retention signals within the marrow, resulting in peripheral neutrophilia. Certain tumor cells can further secrete neutrophilic chemotactic factors, forming a chemokine gradient that guides neutrophils to transmigrate and extravasate into the site of the tumor. Neutrophils that are recruited into the tumor are collectively termed tumor-associated neutrophils (TANs). b) Following natural tumor progression and expansion, cells in the center of a solid tumor are likely to undergo hypoxia and other potential stressors within the tumor microenvironment, resulting in tumor cell/tissue damage. Damaged tumor cells further release damage-associated molecular patterns (DAMPs), which can preferentially recruit neutrophils over other immune cells to tissue damage sites, further augmenting the extent of TANs infiltration. c) As tumor progression advances, oxygen- and nutrient-deprived tumor cells in the center of the solid tumor die, forming a visible tumor necrosis. Dead tumor cells aberrantly upregulate expression and release of a variety of factors, reinforcing bone marrow granulopoiesis and neutrophil release and further magnifying the extent of TANs infiltration within the tumor. Once recruited, TANs can cause additional tumor cell damage/death, augmenting inflammatory responses within the tumor, resulting in further tumor cell/tissue death. Together, these aberrations of marrow granulopoiesis and recruitment of neutrophils into the damaged tumor site facilitated by necrotic tumor cells, as well as TANs-triggered tumor cytotoxicity, form a positive feedback loop, magnifying and accelerating both TANs infiltration and tumor necrosis to their fullest extents.
