Figure 2. Neutrophils can induce tumor cell death via multiple mechanisms.
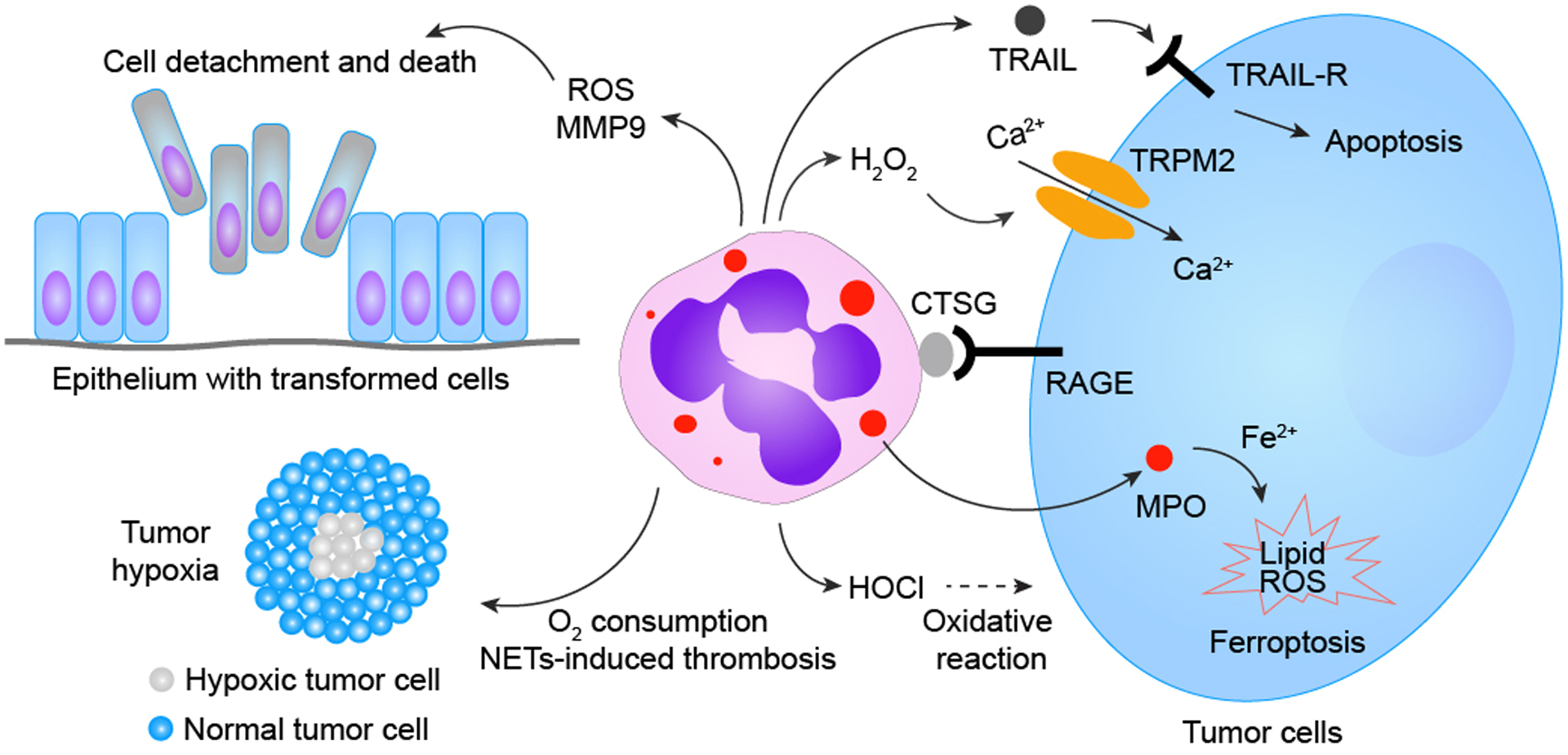
Neutrophils are well-known for their innate capacities to execute oxidative bursts, causing oxidative damage to surrounding cells/tissues. Unsurprisingly, most previously identified neutrophil-triggered tumor cell-killing primarily relies on neutrophils’ production of reactive oxidants. Several byproducts (e.g., hypochlorous acid, HOCl) generated via the neutrophilic MPO−H2O2−Cl− cascade, has been proposed to play a role in neutrophil-mediated tumor cytotoxicity. A study revealed that H2O2 generated in the neutrophilic oxidase cascade can directly instigate tumor cell death via the H2O2-activated nonselective cation channel—transient receptor potential cation channel subfamily M member 2 (TRPM2)—resulting in TRPM2-facilitated uncontrolled Ca2+ influx into tumor cells and subsequent tumor cell death. Neutrophils also execute tumor cytotoxicity via transfer of neutrophilic MPO into tumor cells, which in turn leads to aberrant intracellular accumulation of lipid ROS and eventual tumor cell ferroptosis. Moreover, neutrophils can also generate other tumor-cytotoxic factors, such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), to instigate tumor apoptotic cell death. Neutrophils’ capacities to generate tumor-cytotoxic materials depend on physical contact between neutrophils and tumor cells mediated by neutrophilic cathepsin G (CTSG) and receptor for advanced glycation end products (RAGE). Additionally, neutrophils are capable of triggering tumor cytotoxicity indirectly by causing damage or metabolic disturbances to tumor cells, such as 1) detachment of neoplastically-transformed epithelial cells in a neutrophilic NADPH oxidase-mediated manner, 2) competitive consumption of oxygen in the tumor microenvironment to augment hypoxic stress, and 3) NETs-facilitated vascular damage, leading to thrombosis and obstruction of blood flow, further magnifying hypoxia and nutrient deprivation among tumor cells.
