Fig. 4 |. Addition of armour or subtraction of suppressive genes or their transcripts enables TME resistance.
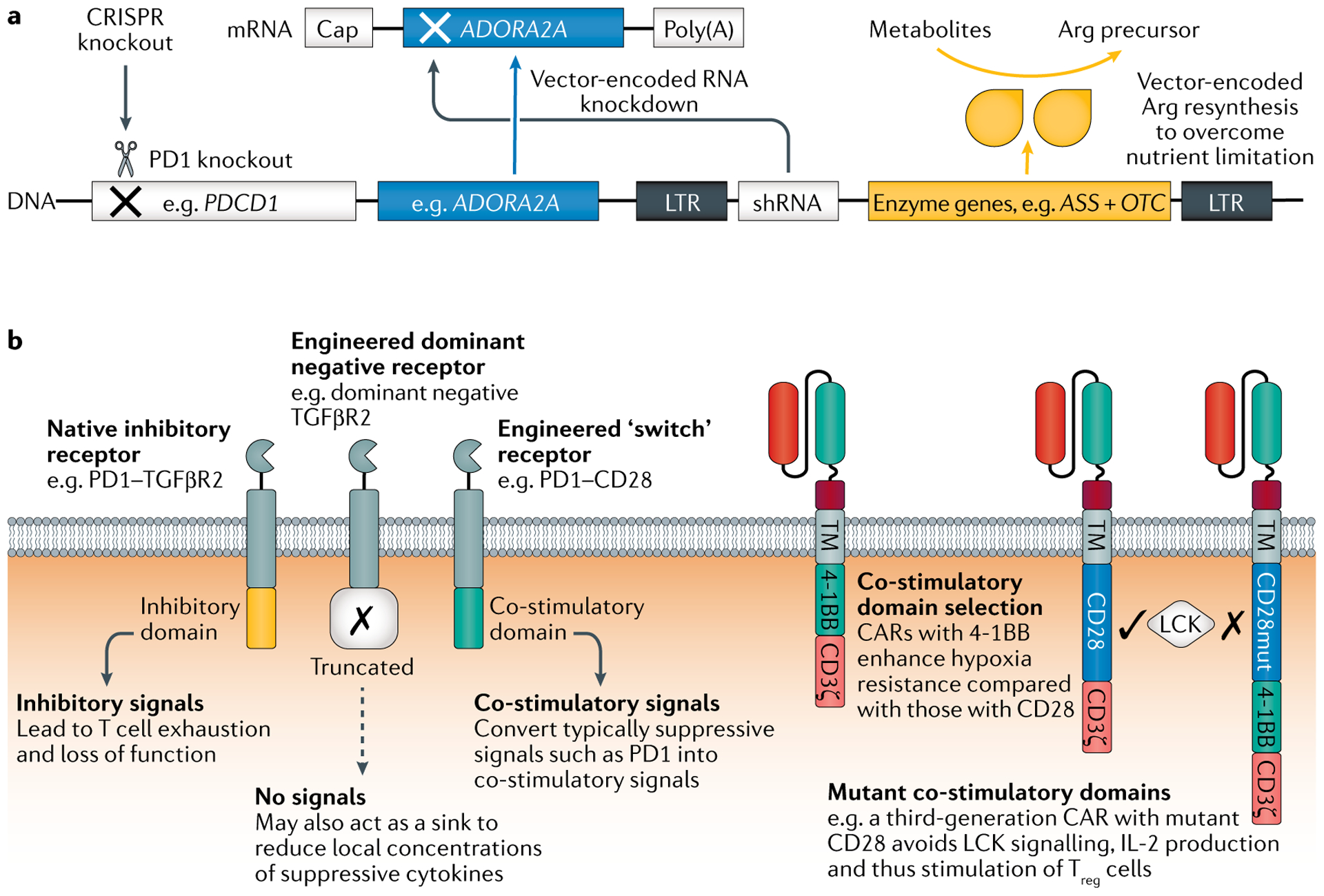
a | Many potential design solutions are available, including gene deletion (for example, using CRISPR–Cas9 genome editing to knock out PDCD1), RNA interference (for example, knockdown of the adenosine A2A receptor gene ADORA2A to confer adenosine resistance) and addition of genes to enable metabolic self-sufficiency (for example, addition of ASS and OTC, which encode the enzymes argininosuccinate synthetase and ornithine transcarbamylase, respectively) or that counter specific checkpoints and suppressive cytokines. b | Receptor-based approaches to resist tumour microenvironment (TME) suppression include expression of a dominant negative form of an inhibitory receptor to act as a sink. Alternatively, the receptor’s inhibitory domain can be switched to a co-stimulatory domain to change the inhibitory signal into an activating one. Chimeric antigen receptor (CAR) design considerations include the choice of co-stimulatory domains; both natural and mutant co-stimulatory domains may enhance the ability of the engineered T cell to survive in the suppressive TME. Multiple strategies may be used in parallel and should be customized to the specific TME roadblocks encountered. LTR, long terminal repeat; shRNA, short hairpin RNA; TM, transmembrane domain; Treg cell, regulatory T cell.
