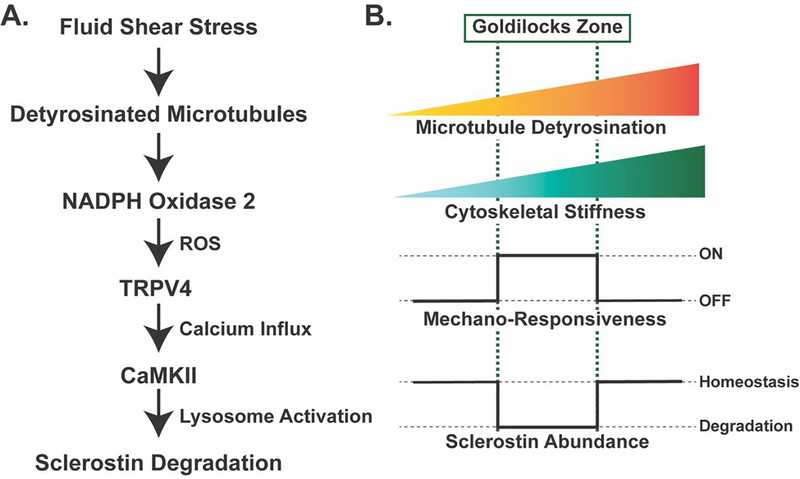
Figure 3: Mechano-sensing in Ocy454 cells through detyrosinated microtubules contributes to the regulated degradation of sclerostin protein and reveals a tunable mechano-sensor by contributing to changes in cytoskeletal stiffness.
A. Fluid shear stress is sensed through a pool of detyrosinated microtubules, which activate NOX2 to produce reactive oxygen species. This reactive oxygen species sensitizes TRPV4 calcium-permeable channels on the cell membrane to allow for calcium influx. Calcium influx activates CaMKII, which activates the rapid degradation of sclerostin protein by the lysosome19,25. B. The level of microtubule detyrosination affects the overall stiffness of the cytoskeleton. Based off in vitro studies in Ocy454 cells, there is a “Goldilocks” level of detyrosination and, in turn, cytoskeletal stiffness, that permits osteocyte mechano-responsiveness, allowing for fluid shear stress to degrade sclerostin protein to reduce its abundance. If detyrosination and cytoskeletal stiffness are above or below this Goldilocks zone, osteocytes are no longer mechano-responsive and sclerostin abundance is unchanged with fluid shear stress19.