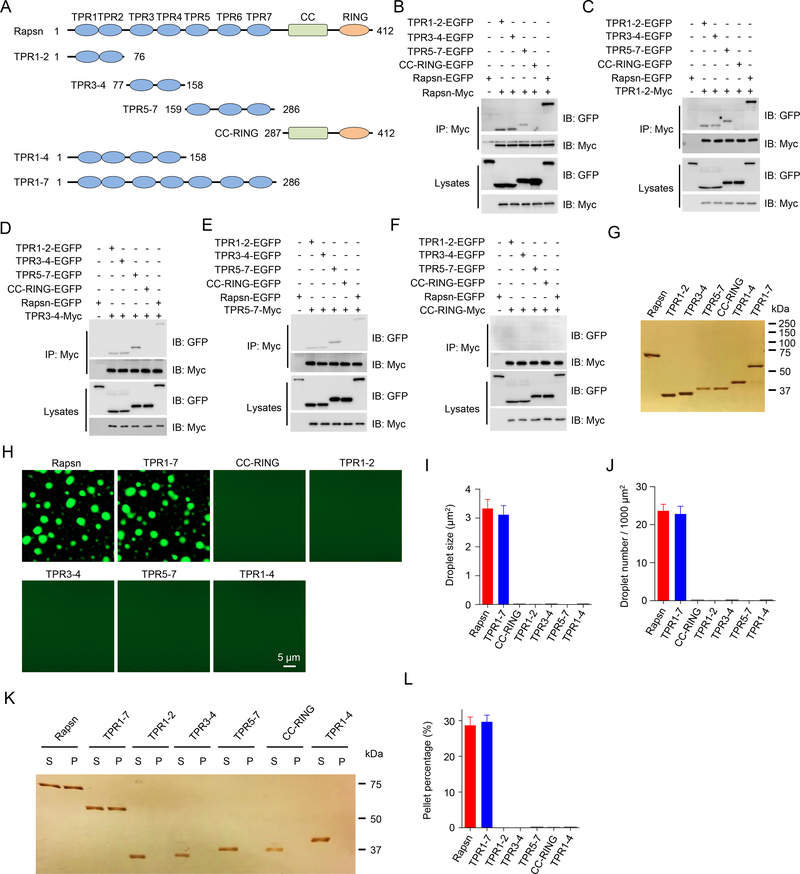
Figure 4. Multivalent binding of TPR domains for Rapsn LLPS.
(A) Schematic domain structures of Rapsn and truncation mutants.
(B) Binding of Rapsn with TPR1–2, TPR3–4, TPR5–7, and full-length Rapsn, but not CC-RING.
(C-F) TPR1–2 (C), TPR3–4 (D), TPR5–7 (E), but not CC-RING (F), were able to self-interact and bind each other.
(G) Silver staining showing purified EGFP-tagged full-length and truncated Rapsn proteins.
(H) LLPS of TPR1–7 and full-length Rapsn, but not CC-RING, TPR1–2, TPR3–4, TPR5–7, and TPR1–4.
(I, J) Quantification of droplet size (I) and number (J) in (H).
(K) Representative silver staining image showing WT and TPR1–7, but not TPR1–2, TPR3–4, TPR5–7, CC-RING, and TPR1–4, were able to condensate into pellets after centrifugation.
(L) Quantification of the percentage of proteins in pellets in (K).
Data was shown as mean ± SEM; n = or > 3.
See also Figure S3.