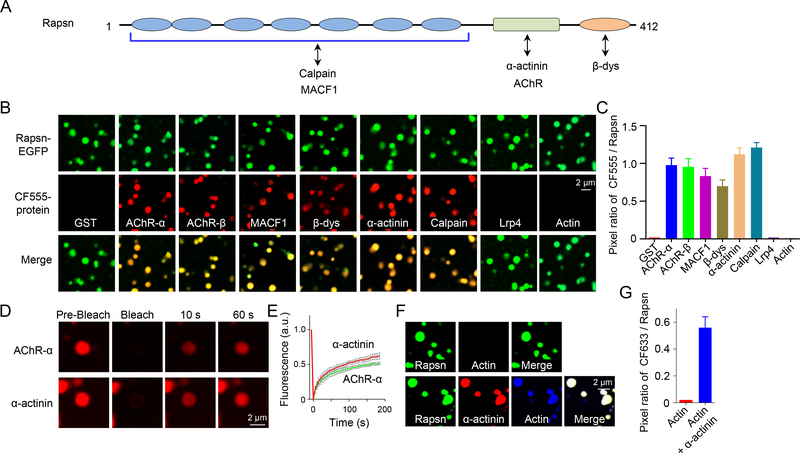
Figure 5. Recruitment of cargo proteins into Rapsn condensates.
(A) Schematic domain structure of Rapsn and interaction proteins.
(B, C) AChR-α, AChR-β, MACF1, β-dystroglycan, α-actinin or calpain, but not GST, LRP4, or actin, were recruited into Rapsn LLPS-mediated condensates.
(D) FRAP analysis of AChR-α and α-actinin enriched in Rapsn droplets showing dynamic protein exchange.
(E) Quantification of fluorescence recovery of AChR-α and α-actinin in (D).
(F) Recruitment of actin into Rapsn condensed droplets in the presence of α-actinin.
(G) Quantification of the pixel ratio of CF633 / Rapsn-EGFP in (F).
Data was shown as mean ± SEM; n = or > 3.
See also Figures S4 and S5.