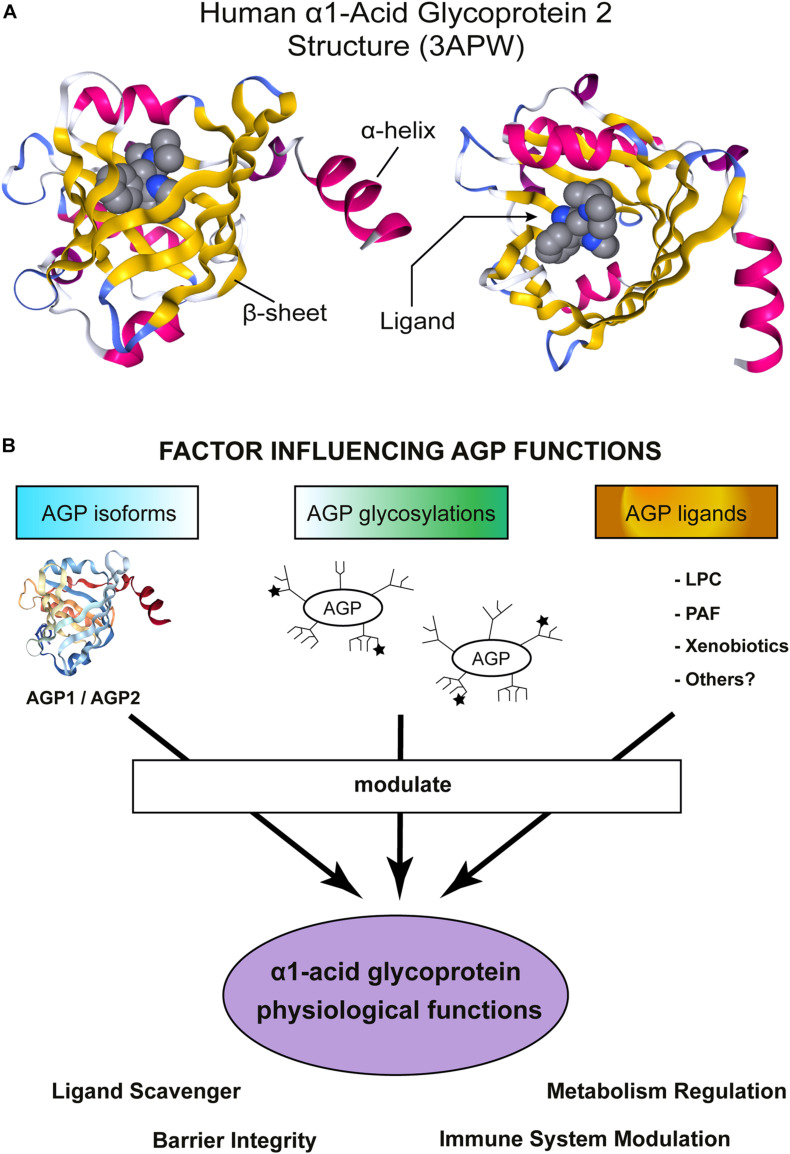
FIGURE 1.
α1-Acid Glycoprotein (AGP) structure and functions. (A) Schematic representation of hAGP2 structure in complex with a drug in the Lipocalin cavity. In this case the ligand is the antiarrhythmic medication disopyramide (3APW) [originaly published by Nishi et al. (2011)]. The left model is a lateral view of AGP2 and the right model show the view from the top, the open side of AGP β-barrel. The secondary structures are colored: β-sheets in yellow and α-helix in magenta. The ligand (disopyramide) is represented in a bulky format to highlight the cavity inside of AGP2. The figure was generating using NGL viewer (Rose et al., 2018). (B) Schematic representation showing important factors influencing AGP functions. AGP is highly heterogenic and numerous activities in vitro and in vivo have been reported. AGP is an acute-phase protein that modulates the immune system and, recently, AGP has also been shown to regulate the metabolism. AGP tissue/cell type of expression determines which isoform is expressed: AGP1 or AGP2. Then, each tissue counts with a specific set of enzymes to glycosylate proteins and, indeed, multiple glycosylation patterns have been detected in AGP. The ★ symbols represent the possible presence of sLex groups. Note that the schematic cartoon of the glycosylation does not represent the actual complex and heterogeneous AGP glycosylation pattern. More detailed information can be found in Baerenfaenger and Meyer (2018), Keser et al. (2021). Finally, the environment where AGP is secreted allows to bind/scavenger a different set of ligands, Indeed, an enormous number of compounds are efficiently bound by AGP. The sum of all the above-mentioned factors would contribute to AGP physiological functions.