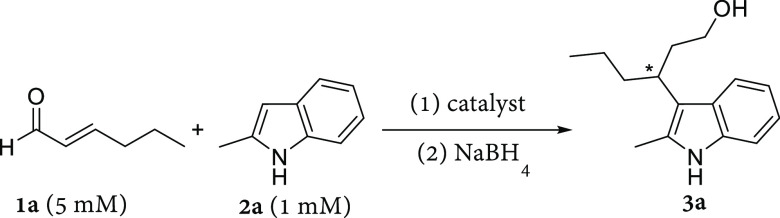
Table 1. Initial Results of LmrR_pAF Catalyzed Formation of 3a from 1a and 2a and Control Reactionsa.
| entry | catalyst | yield (%)b | ee (%)c |
|---|---|---|---|
| 1 | LmrR_pAF (20 μM) | 42 ± 4 | 45 ± 0 |
| 2 | LmrR (20 μM) | 2 ± 0 | –7 ± 0 |
| 3 | LmrR_V15K (20 μM) | 3 ± 0 | –15 ± 0 |
| 4 | LmrR_V15Y (20 μM) | 2 ± 0 | –11 ± 5 |
| 5 | aniline (1 mM) | 8 ± 0 | - |
| 6 | LmrR (20 μM) + aniline (16 μM) | 4 ± 0 | 1 ± 1 |
| 7 | none | <1 | - |
Reaction conditions: LmrR variants (20 μM dimer concentration) in phosphate buffer (50 mM, pH 6.5) containing NaCl (150 mM) and 8 vol % DMF, [2a] = 1 mM, [1a] = 5 mM; reaction time 16 h at 4 °C with mixing by continuous inversion in 300 μL of total volume. Reduction with NaBH4 (60 μL, 20 mg mL–1 in 0.5 w/v% NaOH) afforded the alcohol product 3a. For each entry, two independent experiments were conducted, each in duplicate, except entries 5 and 7 which were conducted in triplicate. Errors are the standard deviation of the results.
Analytical yields determined by normal-phase HPLC (Chiralcel OJ-H) with 3-(3-hydroxypropyl)indole as internal standard.
Enantiomeric excess determined by chiral normal-phase HPLC (Chiralcel OJ-H), sign of the ee value refers to elution order.