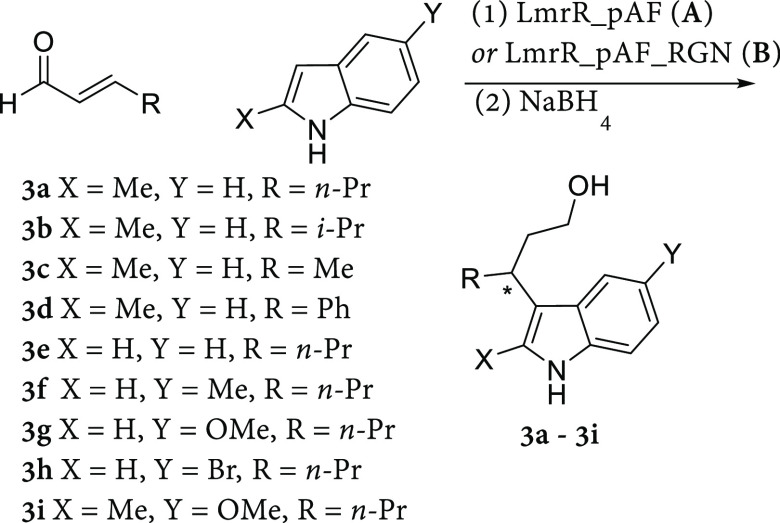
Table 2. Substrate Scope of Indoles and Enals Converted to the Corresponding Friedel–Crafts Products 3a–3i by LmrR_pAF and LmrR_pAF_RGNa.
| entry | product | catalyst | yield (%)b | ee (%)c |
|---|---|---|---|---|
| 1 | 3a | A | 42 ± 4 | 45 ± 0 |
| 2 | 3a | B | 74 ± 2 | 87 ± 0 |
| 3d | 3b | A | 24 ± 1 | 58 ± 0 |
| 4d | 3b | B | 34 ± 6 | 83 ± 2 |
| 5 | 3c | A | 76 ± 7 | 33 ± 1 |
| 6 | 3c | B | 95 ± 7 | 58 ± 1 |
| 7 | 3d | A | 3 ± 0 | 20 ± 1 |
| 8 | 3d | B | 4 ± 1 | 13 ± 9 |
| 9e,f | 3d | LmrR | <1 | - |
| 10g | 3e | A | 10 ± 1 | 50 ± 0 |
| 11g | 3e | B | 27 ± 2 | 89 ± 1 |
| 12g | 3f | A | 16 ± 1 | 41 ± 0 |
| 13g | 3f | B | 31 ± 3 | 89 ± 2 |
| 14g | 3g | A | 24 ± 2 | 55 ± 1 |
| 15g | 3g | B | 40 ± 3 | 89 ± 1 |
| 16f | 3h | A | <1 | - |
| 17f | 3h | B | <1 | - |
| 18 | 3i | A | 62 ± 11 | 58 ± 0 |
| 19 | 3i | B | 75 ± 9 | 82 ± 1 |
| 20g | 3j | A | 21 ± 1 | 66 ± 0 (S)h |
| 21g | 3j | B | 18 ± 2 | 73 ± 1 (S)h |
Reaction conditions: LmrR variants (20 μM dimer concentration) in pH 6.5 buffer containing NaCl (150 mM) and NaH2PO4 (50 mM), 8 vol % DMF, [indole] = 1 mM, [enal] = 5 mM, reaction time 16 h, reactions conducted at 4 °C with mixing by continuous inversion in 300 μL total volume. Reduction with NaBH4 (60 μL, 20 mg mL–1 in 0.5 w/v% NaOH) afforded the alcohol products 3a–3i. For each entry, two independent experiments were conducted, each in duplicate. Errors are the standard deviation of the results thus obtained.
Analytical yields determined using normal phase HPLC using 3-(3-hydroxypropyl)indole as internal standard.
Enantiomeric excess determined using chiral normal-phase HPLC (Chiracel OJ-H (3a), AS-H (3b, 3c, 3f), OD-H (3d, 3e, 3h, 3i, 3j) or OB-H (3g)).
Reaction time 40 h.
LmrR was used in place of LmrR_pAF.
No product could be detected.
50 μM artificial enzyme (concentration of dimer) was used, and reaction time was 64 h.
Absolute configuration asigned by comparison of order of elution on chiral normal-phase HPLC with enantioenriched reference compound and the literature;18 see Supporting Information for details.