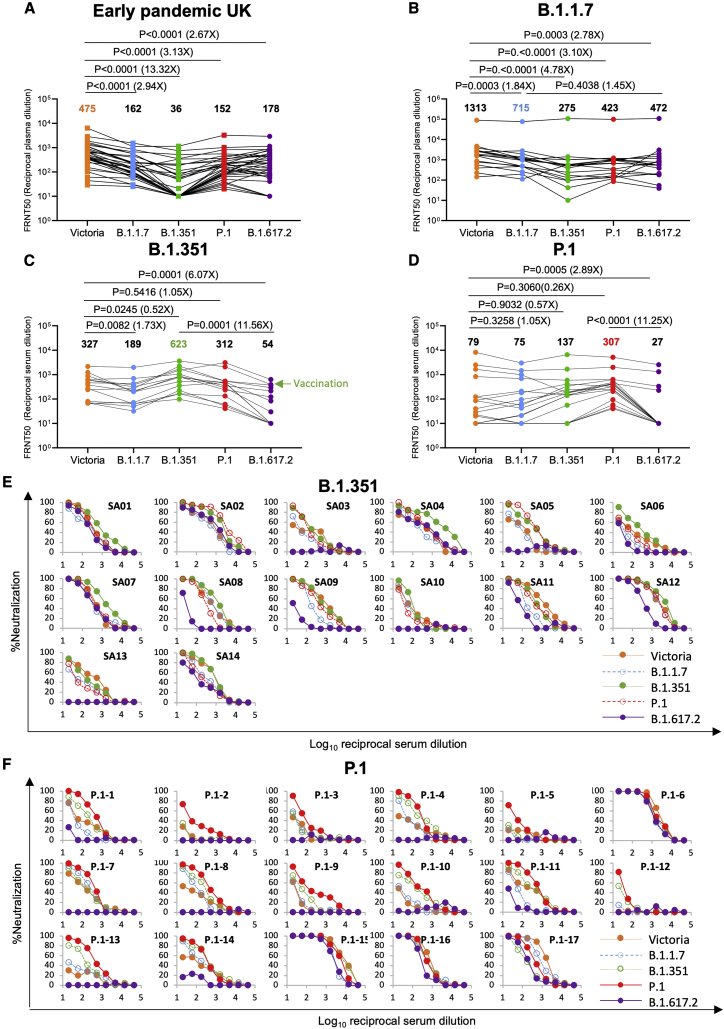
Figure 6.
Neutralization of B.1.617.2 by convalescent plasma
(A) Neutralization of B.1.617.2 live virus, measured by FRNT using the 34 convalescent samples described in Figure 5A; comparison is made with neutralization titers to Victoria, B.1.1.7, B.1.351, and P.1 (filled squares), reported previously in Supasa et al. (2021), Zhou et al. (2021), and Dejnirattisai et al. (2021b), and geometric mean titers are shown above each column.
(B–D) Neutralization titers for Victoria, B.1.1.7, B.1.351, P.1, and B.1.617.2 using (B) B.1.1.7 convalescent plasma, (C) B.1.351 convalescent serum, and (D) P.1 convalescent serum. The green arrow in (C) represents serum from an individual who was infected with B.1.351 and subsequently received a vaccine. Wilcoxon matched-pairs signed-rank test was used for the analysis, and two-tailed p values were calculated. For the data presented for B.1.1.7 in (B), the sample with extremely high titers was excluded from the statistical analysis.
(E and F) Neutralization curves for Victoria, B.1.1.7, B.1.351, P.1, and B.1.617.2 using convalescent serum from (E) B.1.351- and (F) P.1-infected individuals.