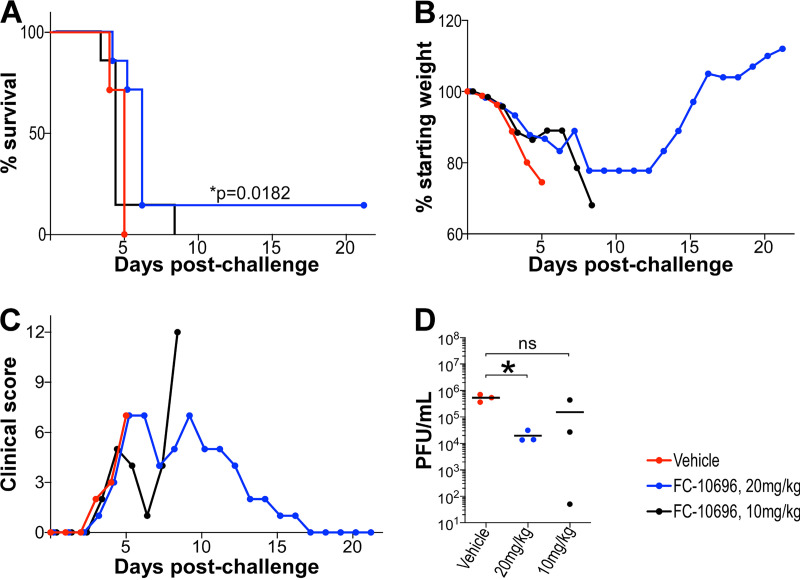
FIG 3.
In vivo efficacy of FC-10696 in a mouse model of MARV disease. Three groups of 10 4-week-old female BALB/cJ mice were challenged with 1,000 PFU of mouse-adapted MARV. Intraperitoneal dosing by vehicle or FC-10696 at either 20 or 10 mg/kg started 6 h postchallenge and continued BID for 10 consecutive days. Animals were observed daily for mortality (A), weight loss (B), and clinical signs of disease (C) for 21 days postinfection. The average weight of animals in each group was determined as the ratio of total weight of all live mice to the number of animals. The percent weight change on each day was determined by dividing the average weight on that day by the average weight on day 0. Clinical scores for each group were recorded as a sum of all observations in the group, and if a score of ≥12 was recorded for an individual animal, it was considered terminally ill and euthanized. On day 3 postchallenge, 3 animals/group were euthanized to collect serum for virus load assessment by the neutral red plaque assay (D). The remaining 7 mice/group were used to determine animal survival. Viral burden was analyzed using a Student's t test or one-way ANOVA with Tukey’s test, and survival analysis was performed using a log-rank (Mantel-Cox) test, with a P value of ≤0.05 considered significant in all analyses.