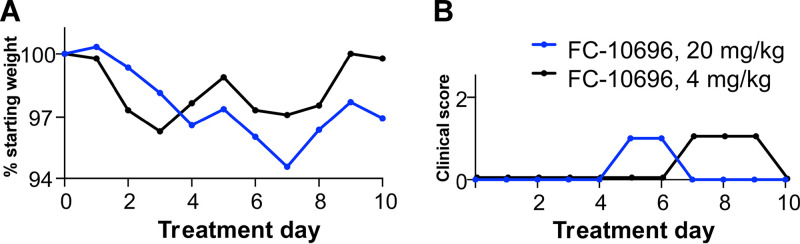
FIG 4.
In vivo toxicity data for FC-10696. FC-10696 was resuspended in a 30% PEG 400–2% DMSO–14% Kleptose HPB parenteral-grade formulation at two different concentrations, 20 and 4 mg/kg, and administered to groups of five 4-week-old female BALB/cJ mice BID via the i.p. route for a period of 10 days. Animals were monitored daily for signs of treatment-associated toxicity: weight loss (A); rough coat, discharge from eyes and nose, diarrhea, and decreased food intake and activity (B); and mortality. The percent weight change in a group on each day was calculated by dividing the average animal weight on that day by the average weight on day 0. Clinical scores for each group were recorded as a sum of all observations in the group.