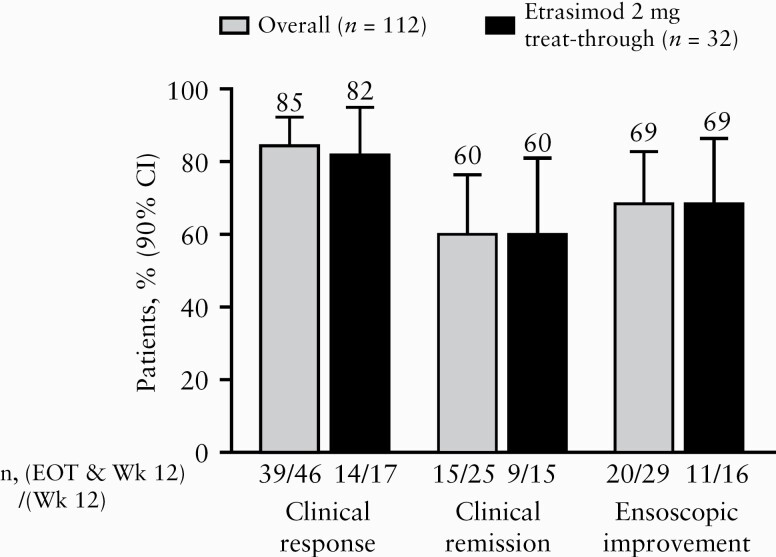
Figure 4.
Proportion of patients with sustained response from Week 12 to EOT [ITT population]. All patients received etrasimod 2 mg during the OLE. The overall group includes patients who received any treatment [placebo, etrasimod 1 mg, or etrasimod 2 mg] during the DB study. The etrasimod 2 mg treat-through group received etrasimod 2 mg during both the DB study and OLE. In these NRI analyses, data missing for any reason were imputed as non-response. CI, confidence interval; DB, double-blind; EOT, end of treatment; ITT, intention-to-treat; n, number of patients; NRI, non-responder imputation; OLE, open-label extension; Wk, week.