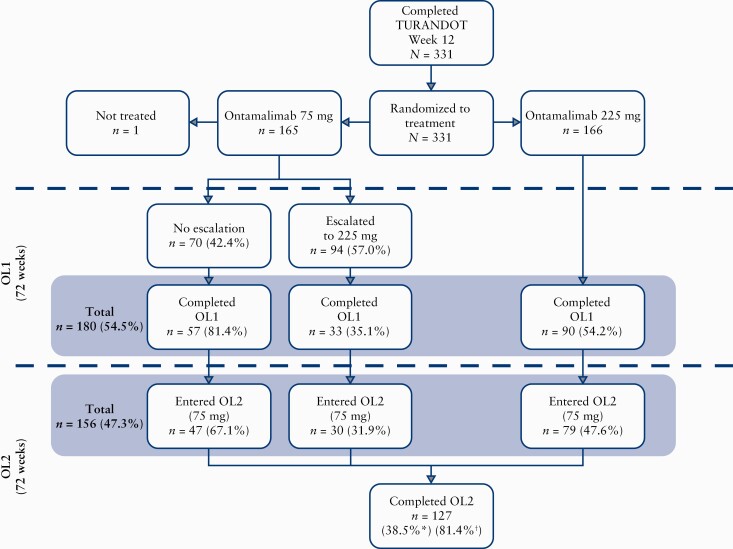
Figure 1.
Patient flow. Of 180 patients who completed OL1, 21 did not enter OL2 and proceeded to follow-up, owing to the timing of a protocol amendment which stipulated that OL2 be added to the study design to further evaluate the long-term safety of ontamalimab. An additional three patients completed OL1 and intended to progress to OL2; however, they proceeded directly to follow-up. OL1, open-label treatment period 1; OL2, open-label treatment period 2. *Calculated as n divided by the number of patients who received any amount of study drug [safety analysis set, n = 330]. †Calculated as n divided by the number of patients who entered OL2 [n = 156].