Abstract
Background: Easyhaler (registered trademark by Orion Corporation) is a multidose dry powder inhaler (DPI) for the treatment of asthma and chronic obstructive pulmonary disease (COPD), designed to be simple and easy to use. Salmeterol–fluticasone propionate (S-F) Easyhaler (50/250 and 50/500 μg per dose), available in several European countries, provides combined inhaled corticosteroid and long-acting beta agonist therapy for the management of asthma and COPD. A requirement of the European Committee for Medical Products for Human Use guidelines is to demonstrate product performance under conditions that mimic real-life patient use. Therefore, our aims were to assess the robustness of the S-F Easyhaler by assessing the delivered dose (DD) and fine particle dose (FPD) throughout the inhaler lifespan and under simulated environmental stress conditions.
Methods: This was a noncomparative exploratory in vitro study. Two batches and six to nine inhalers per batch from both dose strengths were used to assess drug delivery performance over the inhaler lifespan (doses 1–60). For determining the impact of simulated environmental stress (tests for exposure of dropping, vibration, moisture, and freeze–thawing) on DD and FPD, one batch and three inhalers per batch from both dose strengths were used per test, respectively. Aerodynamic particle size distribution was evaluated during the simulated dropping and vibration tests.
Results: DD and FPD from both dose strengths of S-F Easyhaler performance remained consistent through the inhaler lifespan and simulated environmental stress did not affect its performance. Similar DD and FPD values were observed with or without dropping, vibration, exposure to moisture, and freeze–thawing, and no inhaler breakages occurred during the simulated tests.
Conclusions: The in vitro performance of S-F Easyhaler at both dose strengths suggests that reliable dosing and robustness can be achieved under real-life stress conditions; S-F Easyhaler is a durable DPI for the management of asthma and COPD.
Keywords: asthma, COPD, delivered dose, Easyhaler, fine particle dose, salmeterol–fluticasone propionate
Introduction
Inhaled medications are a mainstay of treatment in patients experiencing chronic respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD).(1,2) The inhaled route of administration offers a number of advantages, such as direct delivery of the drug in high concentrations to the lung, rapid onset of action, and minimal systemic side effects.(3)
Dry powder inhalers (DPIs) are among the most widely used inhaler devices currently available.(4) To provide sufficient benefit, DPIs should perform consistently during repeated use, by delivering a predictable drug dose in a reproducible manner throughout the inhaler lifespan.(5,6) In addition, inhaled particles should have an aerodynamic size of ≤1–5 μm (taking 0.5 μm particle evacuation into account), enabling penetration beyond the upper airways and allowing optimal lung deposition.(7) Robustness of inhaler performance and consistent functioning under different environmental conditions are further key requirements for DPIs.(6) Several factors could potentially affect the uniformity of the delivered dose (DD) or fine particle dose (FPD) of DPIs, including those associated with storage or transportation (e.g., fluctuations in temperature or humidity and/or vibration)(6,8) and patient rough handling (e.g., dropping).(6)
Combined therapy with an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA) is recommended as part of a stepwise approach to the treatment of patients with asthma and COPD.(1,9) The ICS/LABA combination is an initial treatment option for patients with troublesome asthma symptoms, and those presenting with severely uncontrolled asthma or an acute exacerbation.(9)
The Easyhaler (registered trademark by Orion Corporation) is a reservoir-based multidose DPI (Fig. 1) that enables the administration of several inhaled single or combination pharmacotherapies, including the ICS/LABA products, budesonide–formoterol (B-F) and salmeterol–fluticasone propionate (S-F).(5,10–12) The S-F Easyhaler was granted approval in 2018 for the treatment of patients with asthma and COPD in nearly all European countries. S-F Easyhaler is available in two dose strengths, 50/250 (S-F) and 50/500 μg per dose, taken as a single inhalation twice daily. The dose should be titrated to the lowest dose at which effective control of symptoms is maintained.(10–12)
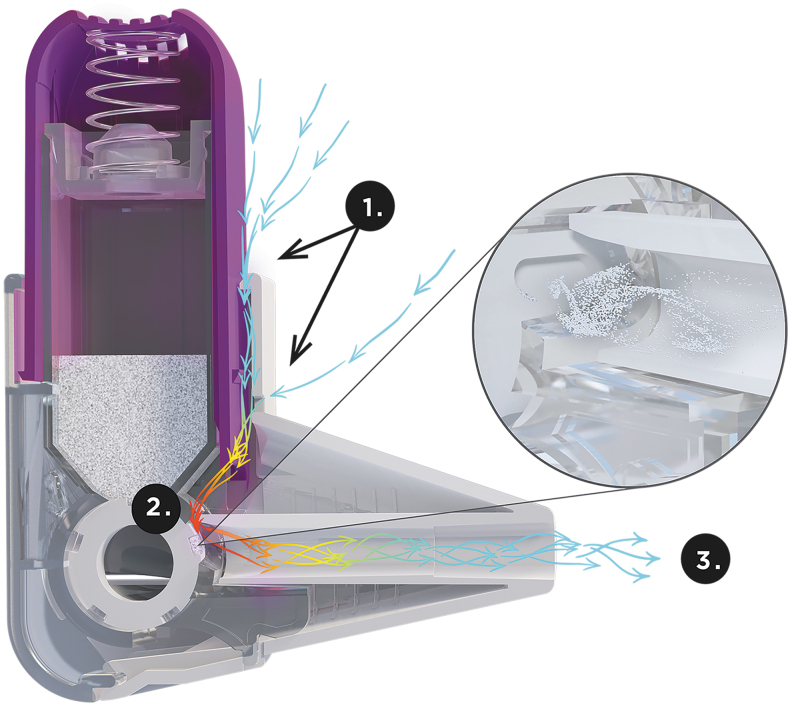
FIG. 1.
Cross-sectional diagram of the Easyhaler. When the patient inhales, air enters the Easyhaler around the actuator and encounters high or medium-to-high resistance due to the small size of the air vent (1); the resistance generates turbulent air flow to the dosing cup (2); turbulent air flow ensures deaggregation of drug particles and high dose emissions, even with low patient inhalation flows (3).
Approval of the S-F Easyhaler was based on demonstrations of therapeutic equivalence in comparison with the reference combination of ICS/LABA DPI (Seretide Diskus®; GlaxoSmithKline, Brentford, United Kingdom), in accordance with European Union's Committee for Medicinal Products for Human Use (CHMP) guidelines.(6,13) Both S-F Easyhaler and Seretide Diskus are breath-actuated multidose DPIs and they contain 60 doses. Diskus in a disk-shaped inhaler with a 60 doses premetered drug blister strip. Device-metered Easyhaler with its elongated mouthpiece shares the same functional form as common metered dose inhalers. In a randomized crossover pharmacokinetic study in healthy volunteers (N = 65), S-F Easyhaler demonstrated bioequivalence with Seretide Diskus, with regard to lung deposition applying charcoal block in the pharmacokinetic (PK) study and total systemic exposure of S-F.(14) In a two-part study, an initial randomized controlled trial (RCT) assessed inspiratory flow parameters of the S-F Easyhaler and Seretide Diskus in subgroups of patients with asthma (children, adolescents, and adults, including elderly patients) and patients with COPD (N = 227). In the subsequent in vitro study using clinically relevant airflow rates derived from the RCT, the S-F Easyhaler and Seretide Diskus showed similar flow rate dependence of the DD and FPD across a range of clinically relevant airflow rates.(6,13,15) Both devices had medium air flow dependency based on Q-index (15%–40%).(15,16)
A key requirement of CHMP guidance is that product performance should be investigated under conditions that simulate patient use, at the same frequency as indicated in the product label. Based on the regulatory requirements, the aims of this exploratory study were to assess whether the DD and FPD of the S-F Easyhaler remained consistent throughout the inhaler lifespan and under simulated environmental stress conditions, modeled using a variety of in vitro environmental stress tests.
Materials and Methods
Study design
This noncomparative exploratory in vitro study evaluated whether the dosing properties (DD and FPD) of the 50/250 and 50/500 μg per dose S-F Easyhaler remain consistent throughout the inhaler lifespan, and when subjected to various simulated stress tests, including the effects of dropping, vibration, moisture, and freeze–thawing, to determine the robustness of the inhaler. Aerodynamic particle size distribution (APSD) was also evaluated following the dropping and vibration simulated test conditions.
All assessments were performed in accordance with guidelines for in vitro testing established by the European Pharmacopoeia (edition 9.0).(17)
Devices and reagents
All inhalers were provided by Orion Pharma, Orion Corporation, Finland. As suggested in the CHMP guidance on the pharmaceutical quality of inhalation and nasal products(6) for the testing of drug delivery performance over the inhaler lifespan, two batches and six to nine new inhalers per batch from both dose strengths were used. When conducting the simulated stress tests to examine the robustness of the S-F Easyhaler, one batch and three new inhalers per batch from each dose strength were used for determination of DD, FPD, and APSD, respectively.
Assessments
Delivered dose
The sampling apparatus and procedures defined in the European Pharmacopoeia 9.0(17) were used to determine the DD.(15,17) The flow rate and corresponding pressure drop used to draw air through the inhaler and the sample dissolution method used follow those described by Jõgi et al.(15) In brief, 4 L of air based on European requirements was drawn through the inhaler at a flow rate corresponding to a 4 kPa pressure drop across the inhaler. The amount of active drug collected into the sampling apparatus was determined by high performance liquid chromatography (HPLC), as described earlier.(15)
APSD and FPD
APSD was determined using a next-generation impactor (NGI) equipped with a preseparator, in accordance with the European Pharmacopoeia 9.0.(15,17) Each analysis of APSD, including mass median aerodynamic diameter (MMAD), stage-by-stage, and the quantitative recovery of S-F, was performed as previously described,(15) with 10 doses based on drug substance assay sensitivity were discharged into the NGI at a flow rate (∼55 L/min) corresponding to a 4 kPa pressure drop across the Easyhaler inhaler that has airflow resistance of 0.036 √kPa·min/L.(15) From APSD's stage-by-stage data, the FPD (<5 μm) was calculated using the NGI stage cutoff diameters, established based on the inhaler's characteristic resistance and the methodology described for apparatus E (NGI) in the European Pharmacopoeia.(17)
Lifespan of the inhaler
Determination of DD and FPD through the lifespan of the inhaler was performed in a 1-month study, during minimum dosing intervals, that is, twice-daily dosing. Analyses of DD and FPD were performed at the beginning, middle, and end of the labeled number of doses for each dose strength of the S-F Easyhaler, equating to doses 1–5, 25–29, and 56–60 for assessments of DD and doses 1–10 and 51–60 for assessments of FPD, throughout the inhaler lifespan.
Simulated stress test conditions used to evaluate DD, FPD, and particle size distribution
For all tests described hereunder, one batch per dose strength and three new inhalers per batch were used based on CHMP guidance on the pharmaceutical quality of inhalation and nasal products.(6)
Dropping
Inhalers were dropped onto a wooden surface from a height of 1 m, according to International Organization for Standardization 20072:2009 testing standards.(18) Measurements were taken at the beginning (doses 1–5 before drop, doses 6–10 after the drop for DD and doses 1–10 before drop, doses 11–20 after the drop for FPD) and end (doses 51–55 before drop, doses 56–60 after the drop for DD and doses 41–50 before drop, doses 51–60 after the drop for FPD) of the labeled number of doses, comparing DD and FPD results before and after dropping. Measurements of DD and FPD were performed as previously described.(5)
Vibration
Vibration stress tests were performed according to International Electrotechnical Commission guidance on environmental testing IEC 60068-2-64:2008,(19) and as described by Haikarainen et al.(5) Based on the standard, the inhalers were vibrated in the vertical axis for 60 minutes, using the following parameters: frequency range of 5–500 Hz, acceleration spectral density (ASD) level 1 m2/s3 5–20 Hz, ASD level 3 decibels/octave 20–500 Hz, total spectral acceleration 0.9 g, and uncertainty of measurements 5%.
Moisture
The effect of moisture was assessed by placing inhalers (removed from their aluminum laminate pouch) under storage conditions of 30°C/75% relative humidity (RH) for 48 h. As per the methods described by Haikarainen et al.,(5) DD and FPD were evaluated from the first 5 and 10 doses, respectively, before application of storage conditions; after storage, DD and FPD were analyzed from the next 5 and 10 doses in ambient laboratory conditions, respectively.
Freeze–thawing
Freeze–thawing was performed as previously described,(5) by placing inhalers (both within and removed from their aluminum laminate pouch simulating storage, transportation, and carrying inhaler) at −20°C (±5°C) for 2–3 days, before moving them to an elevated temperature (25°C ± 2°C) in an atmosphere of 60% ± 5% RH for 2–3 days, and repeating this for a total of three cycles. This process was carried out for 2 weeks, after which DD and FPD were subsequently analyzed and compared with those of reference inhalers not subjected to freeze–thawing, but that had been sealed inside their aluminum laminate pouch and kept at 25°C in an atmosphere of 60% RH for the same duration.
HPLC quantitation of S-F
Amounts of S-F were determined from all samples using validated HPLC, as previously described.(15)
Statistical analyses
All analyses were performed as described earlier, with DD and FPD data obtained from the S-F Easyhaler presented as mean values with standard deviation; results are expressed as a percentage, with deviations from 100 indicating the difference compared with the reference value. The NGI results of dropping and vibration tests were also expressed as μg/inhalation for each stage.(15)
All analyses were performed using Microsoft Excel (Office Professional Plus 2016; Microsoft Corporation, Redmond, Washington).
Results
DD and FPD over the S-F Easyhaler lifespan
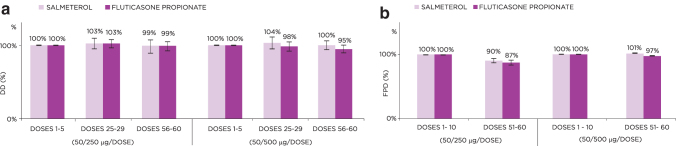
For both the 50/250 and 50/500 μg per dose strengths, DD remained stable throughout the lifespan of the S-F Easyhaler, ranging from 99% to 104% for salmeterol and from 95% to 103% for fluticasone propionate at the middle and end of the labeled doses, when setting the initial dose values to 100% (Fig. 2a). Measured FPD was also similar at the beginning and end of the inhaler lifespan for both dose strengths (Fig. 2b).
FIG. 2.
DDs (a) and FPD values (b) for the 50/250 and 50/500 μg S-F Easyhaler from the beginning through to the end of the inhaler lifespan. Data represent average value ± standard deviation. DD, delivered dose; FPD, fine particle dose; S-F, salmeterol–fluticasone propionate.
MMAD, mass balance, and impaction parameter
The MMAD for S-F Easyhaler was ∼2.82 μm for fluticasone and 2.55 μm for salmeterol. The mass balance for salmeterol and fluticasone was 98%. The impaction parameter(20,21) da2Q was 440 μm2 L/min for fluticasone and 360 μm2 L/min for salmeterol. In the impaction parameter calculation, the flow value of 55 L/min was used.
Impact of simulated environmental stresses on DD and FPD from the S-F Easyhaler
Dropping
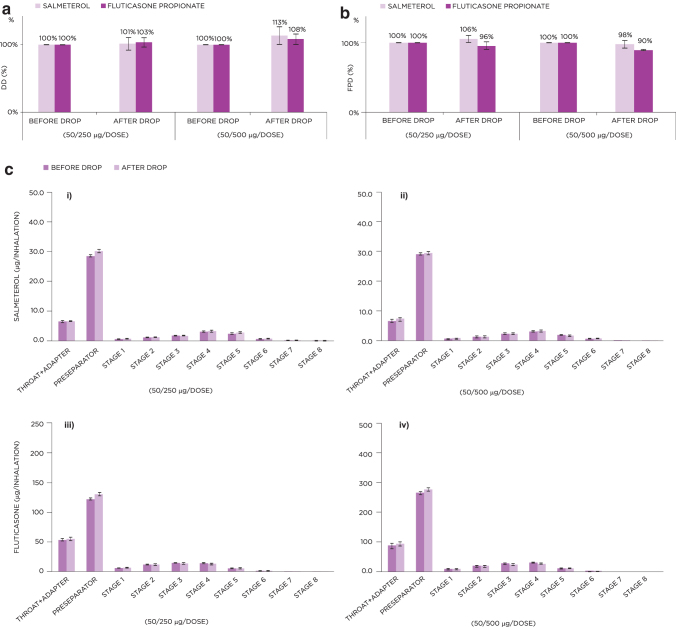
Dropping did not decrease the DD from the S-F Easyhaler compared with DD values assessed before dropping (Fig. 3a). The impact on FPD was minor, with values for S-F assessed as 98%–106% and 90%–100%, respectively, after dropping, compared with the reference values of 100% without dropping (Fig. 3b). No inhaler breakages occurred after dropping stress tests.
FIG. 3.
Effect of dropping on the DD (a) and FPD (b) for the 50/250 and 50/500 μg S-F Easyhaler. (c) Shows NGI stage-by-stage data of APSD results for the 50/250 (i, iii) and 50/500 μg (ii, iv) S-F Easyhaler, before and after the inhaler was dropped. Data represent average value ± standard deviation. APSD, aerodynamic particle size distribution; NGI, next-generation impactor.
Stage-by-stage analysis of APSD using the NGI confirmed that dropping did not markedly influence the performance of either the 50/250 or 50/500 μg per dose S-F Easyhaler DPIs; data at all stages were comparable before and after S-F Easyhaler exposure to these simulated test conditions (Fig. 3c).
Vibration
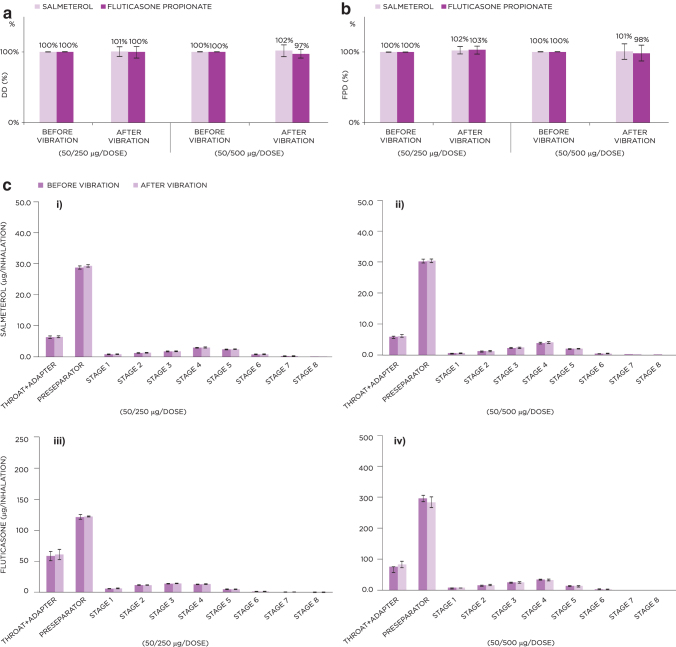
Vibration did not have a major effect on DD or FPD from either dose of S-F Easyhaler, with assessed values after vibration being comparable with those of the reference inhalers without vibration (DD: 100%–102% and 97%–100%; FPD: 100%–102% and 98%–103% across all “before and after” vibration assessments, for S-F, respectively) (Fig. 4a, b). No inhaler breakages occurred after vibration stress tests.
FIG. 4.
Effect of vibration on the DD (a) and FPD (b) for the 50/250 and 50/500 μg S-F Easyhaler. (c) Shows NGI stage-by-stage data of APSD results for the 50/250 (i, iii) and 50/500 μg (ii, iv) S-F Easyhaler before and after vibration. Data represent average value ± standard deviation.
Similar to the dropping test results, stage-by-stage APSD data suggest that no marked influence on performance was observed before or after vibration (Fig. 4c).
Moisture
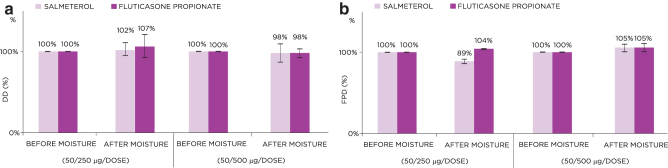
DD from the S-F Easyhaler was unaffected by exposure to moisture, with only minor deviations from the pre-exposure DD values observed after moisture exposure (98%–102% and 98%–107% for salmeterol and fluticasone propionate, respectively) (Fig. 5a). Similarly, FPD was not markedly affected by moisture (postexposure values for S-F: 89%–105% and 104%–105%, respectively, versus pre-exposure values set to 100%; Fig. 5b).
FIG. 5.
Effect of moisture on the DD (a) and FPD (b) for the 50/250 and 50/500 μg S-F Easyhaler. Data represent average value ± standard deviation.
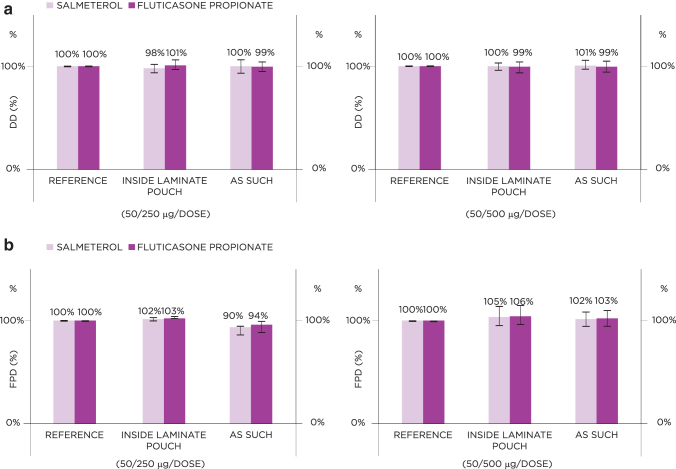
Freeze–thawing
Freeze–thawing did not affect the DD and FPD of the S-F Easyhaler; compared with reference inhaler values set to 100%, DD values ranged from 98% to 100% (inhaler tested inside aluminum laminate pouch) and from 100% to 101% (inhaler tested without aluminum laminate pouch) for salmeterol, and from 99% to 101% (for inhalers tested both inside and outside of the aluminum laminate pouch) for fluticasone propionate (Fig. 6a). Corresponding FPD values ranged from 102% to 105% (inhaler tested inside aluminum laminate pouch) and from 90% to 102% (inhaler tested without aluminum laminate pouch) for salmeterol, and from 103% to 106% (inhaler tested inside aluminum laminate pouch) and from 94% to 103% (inhaler tested without aluminum laminate pouch) for fluticasone propionate (Fig. 6b).
FIG. 6.
Effect of freeze–thawing on the DD (a) and FPD (b) for the 50/250 and 50/500 μg S-F Easyhaler. Data represent average value ± standard deviation.
Discussion
This in vitro exploratory study confirmed the reliability and consistent dosing of the 50/250 and 50/500 μg per dose S-F Easyhaler DPIs throughout the inhaler lifespan, and provided an indication of their robustness in real-life use, as demonstrated by the negligible impact of dropping, vibration, exposure to moisture, and freeze–thawing on inhaler performance and functioning. NGI-based stage-by-stage analysis of APSD indicated that negative segregation of fine particles does not occur in typical handling of the S-F Easyhaler. These results fulfill the requirements to investigate product performance under conditions that simulate environmental stresses, which are stated in the CHMP guideline on pharmaceutical quality of inhalation and nasal products,(6) and which underlie regulatory approval of the S-F Easyhaler in Europe.
The findings of this study complement and build on those observed in a previous evaluation of the B-F Easyhaler (applies the same Easyhaler device), which demonstrated similar consistency in DD, FPD, and APSD over the inhaler lifespan and in response to similar simulated environmental stress testing.(5) In that study, the consistency of dosing of the Easyhaler was also found to be superior to that of the first ICS/LABA combination inhaler available on the market in Europe, the Symbicort® Turbuhaler® (AstraZeneca, Cambridge, United Kingdom).(5) The current combined body of evidence on the robustness of the Easyhaler DPI shows that it has a high tolerance for simulated environmental stress and suboptimal handling, with almost negligible impacts on DD and FPD. This should reassure patients with asthma and COPD that the S-F and B-F Easyhalers will perform predictably and reliably throughout the whole inhaler lifespan, irrespective of typical user handling and storage and transport conditions, thus ensuring effective management of disease symptoms.
A recent article by Weers et al. discussed how to bypass deposition in upper respiratory track (URT) deposition.(21) In addition to benefits of higher air flow resistance for reducing air flow and optimized mouthpiece that enables bypassing teeth and tongue,(15,21) Weers et al. bring up possibilities reducing aerodynamic diameter of the particles for optimizing MIPP further.(21) The large porous particles (low aerodynamic particle size) combined with high resistance inhaler could potentially reduce the URT <2% with MIPP level <150 μm2·L/min and reduce the product's airflow dependency neglible.(21)
A second entry product such as S-F Easyhaler in the first place needs to be adapted to the originator product's pharmacokinetic performance and, therefore, not all of the development options can be used.(13) Easyhaler has design differences compared with Diskus, for example, higher air flow resistance and the elongated mouthpiece.(15) The Easyhaler (EH) formulation is optimized for operation similar to Diskus.
Our results further support the earlier findings that S-F Easyhaler meets several of the typical characteristics of an ideal inhaler, as described by Lavorini.(21,22) These are earlier highlighted elsewhere and they include effectiveness in ensuring inhalation of an adequate drug fraction in appropriately sized particles for lung deposition, largely independent of changes in patient inspiratory flow(15,22,23); ability to demonstrate high reproducibility and consistency(5,21,24); precision(6,8); stability(6,8); and versatility in enabling the administration of different drugs.(8,25) For these reasons, the S-F Easyhaler can be considered a convenient and appropriate inhaler for day-to-day use in patients with asthma and COPD.
The study was carried out according to current standard pharmacopeial testing methodology, complied with accepted guidelines for assessment of orally inhaled products.(6,13,17,26) The study did not include a comparator DPI, but a recent study of the performance and robustness of the B-F Easyhaler demonstrated superiority in dosing consistency of this DPI over the Symbicort Turbuhaler.(5) Although this study employed standard in vitro testing procedures under exposure to environmental stress conditions, such methods are not able to emulate and account for all scenarios. More realistic in vitro testing methods are now available that can mimic real-life lung deposition more accurately, such as those involving anatomically correct throat models and realistic patient flow profiles, and it would be of interest in the future to re-examine some of our findings using these advanced methods.
In conclusion, the 50/250 and 50/500 μg per dose S-F Easyhaler DPIs are able to deliver consistent and reliable dosing throughout the lifespan of the inhaler, and irrespective of simulated environmental stress and/or suboptimal handling. They meet European regulatory criteria for orally inhaled product devices and provide a suitable therapeutic option for the treatment of asthma and COPD. For patients with asthma and COPD, S-F Easyhaler allows a safe and effective choice.
Acknowledgments
Editorial assistance in the preparation of this article was provided by David Griffiths, PhD, CMPP of Bioscript Medical, funded by Orion Corporation, Orion Pharma.
Author Disclosure Statement
All authors are employees of Orion Corporation, Orion Pharma.
Funding Information
This study was funded by Orion Corporation, Orion Pharma.
Reviewed by:
Nani Kadrichu
Denise Conti
References
- 1. Global Initiative for Asthma: Global strategy for asthma management and prevention. 2019. https://ginasthma.org/reports/2019-gina-report-global-strategy-for-asthma-management-and-prevention (accessed September2020)
- 2. Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2019 report. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf/ (accessed September2020)
- 3. Moroni-Zentgraf P, Usmani OS, and Halpin DMG: Inhalation devices. Can Respir J. 2018;2018:5642074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lavorini F, Pistolesi M, and Usmani OS: Recent advances in capsule-based dry powder inhaler technology. Multidiscip Respir Med. 2017;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haikarainen J, Selroos O, Löytänä T, Metsärinne S, Happonen A, and Rytilä P: Budesonide/Formoterol Easyhaler®: Performance under simulated real-life conditions. Pulm Ther. 2017;3:125–138 [Google Scholar]
- 6. Committee for Medicinal Products for Human Use: Guideline on the pharmaceutical quality of inhalation and nasal products. 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003568.pdf/ (accessed January2019)
- 7. Laube BL, Janssens HM, de Jongh FH, Devadason SG, Dhand R, Diot P, Everard ML, Horvath I, Navalesi P, Voshaar T, and Chrystyn H: What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–1331 [DOI] [PubMed] [Google Scholar]
- 8. Chrystyn H: Closer to an ‘ideal inhaler’ with the Easyhaler: An innovative dry powder inhaler. Clin Drug Investig. 2006;26:175–183 [DOI] [PubMed] [Google Scholar]
- 9. Global Initiative for Asthma: Global strategy for asthma management and prevention (2018 update). https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf/ (accessed June2020)
- 10. Orion Pharma (UK) Limited: Fusacomb Easyhaler 50/250 inhalation powder summary of product characteristics. 2018. https://www.medicines.org.uk/emc/product/9224/smpc (accessed July2019)
- 11. Orion Pharma (UK) Limited: Fusacomb Easyhaler 50/500 inhalation powder summary of product characteristics. 2018. https://www.medicines.org.uk/emc/product/9226/smpc (accessed July2019)
- 12. European Medicines Compendium: List of products for Orion Pharma Ltd. https://www.medicines.org.uk/emc/company/99/ (accessed January2019)
- 13. Committee for Medicinal Products for Human Use: Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents. 2009. https://www.ema.europa.eu/documents/scientific-guideline/guideline-requirements-clinical-documentation-orally-inhaled-products-oip-including-requirements_en.pdf (accessed January2019)
- 14. Kirjavainen M, Mattila L, Vahteristo M, Korhonen J, and Lahelma S: Pharmacokinetics of salmeterol and fluticasone propionate delivered in combination via easyhaler and diskus dry powder inhalers in healthy subjects. J Aerosol Med Pulm Drug Deliv. 2018;31:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jõgi R, Lahelma S, Vahteristo M, Happonen A, and Haikarainen J: In vitro flow rate dependency of delivered dose and fine particle dose of salmeterol/fluticasone propionate easyhaler and seretide diskus with patient flow rates collected in a randomized controlled trial. J Aerosol Med Pulm Drug Deliv. 2019;32:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weers J, and Clark A: The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharm Res. 2017;34:507–528 [DOI] [PubMed] [Google Scholar]
- 17. European Pharmacopoeia: 9th ed. 2.9.18. Preparations for Inhalation: Aerodynamic Assessment of Fine Particles. Council of Europe, 2017
- 18. International Organization for Standardization: ISO 20072:2009 standard. Aerosol drug delivery device design verification—requirements and test methods. https://www.iso.org/standard/41989.html (accessed July2019)
- 19. International Electrotechnical Commission: IEC 60068-2-64:2008. Environmental testing—Part 2-64: Tests—Test Fh: vibration, broadband random and guidance. https://webstore.iec.ch/publication/65892/ (accessed July2019)
- 20. Stahlhofen W, Rudolf G, and James AC: Intercomparison of experimental regional aerosol deposition data. J Aerosol Med. 1989;2:285–308 [Google Scholar]
- 21. Weers JG, Son YJ, Glusker M, Haynes A, Huang D, Kadrichu N, Le J, Li X, Malcolmson R, Miller DP, Tarara TE, Ung K, and Clark A: Idealhalers versus realhalers: Is it possible to bypass deposition in the upper respiratory tract? J Aerosol Med Pulm Drug Deliv. 2019;32:55–69 [DOI] [PubMed] [Google Scholar]
- 22. Lavorini F: Easyhaler®: An overview of an inhaler device for day-to-day use in patients with asthma and chronic obstructive pulmonary disease. Drugs Context. 2019;8:212596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abadelah M, Hazim F, Chrystyn H, Bagherisadeghi G, Rahmoune H, and Larhrib H: Effect of maximum inhalation flow and inhaled volume on formoterol drug deposition in-vitro from an Easyhaler(R) dry powder inhaler. Eur J Pharm Sci. 2017;104:180–187 [DOI] [PubMed] [Google Scholar]
- 24. Bjermer L: The importance of continuity in inhaler device choice for asthma and chronic obstructive pulmonary disease. Respiration 2014;88:346–352 [DOI] [PubMed] [Google Scholar]
- 25. Lavorini F, Magnan A, Dubus JC, Voshaar T, Corbetta L, Broeders M, Dekhuijzen R, Sanchis J, Viejo JL, Barnes P, Corrigan C, Levy M, and Crompton GK: Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102:593–604 [DOI] [PubMed] [Google Scholar]
- 26. The United States Pharmacopoeia Convention: USP 35: Physical Tests and Determinations—<601> Aerosols, nasal sprays, metered dose inhalers, and dry powder inhalers. 2012. www.triphasepharmasolutions.com/Private/USP%20601%20AEROSOLS,%20NASAL%20SPRAYS.pdf (accessed July2019)