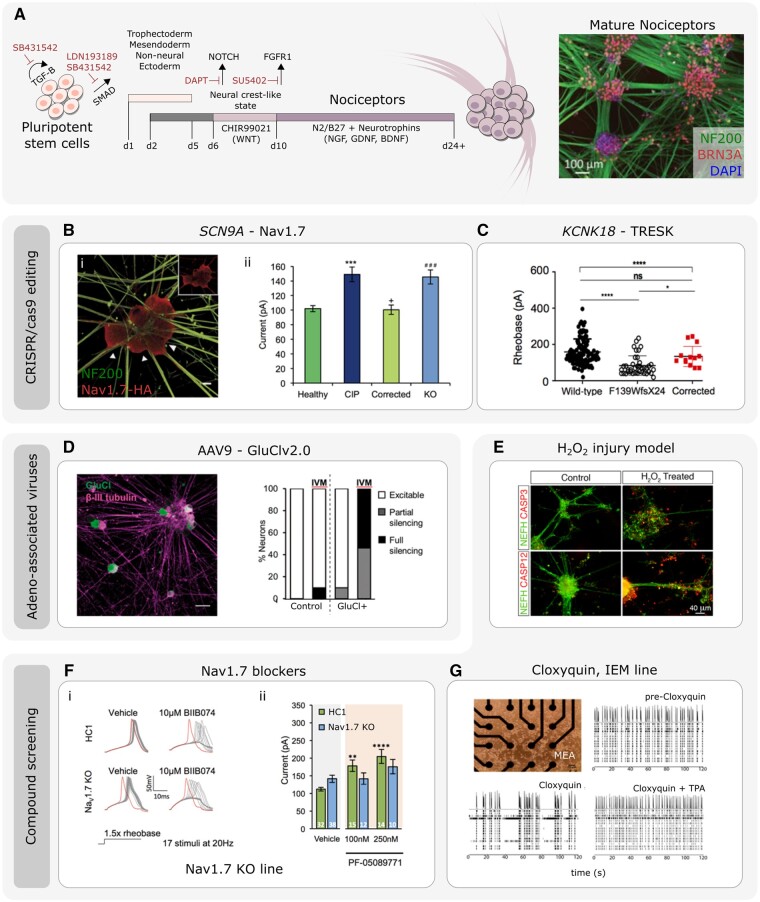
Figure 4.
Using IPSC-derived nociceptors to develop therapeutics for pain disorders. (A) Illustration of the protocol and timeline of events to generate mature IPSC-derived nociceptors (adapted from Chambers et al.102 and Meentz et al.104; immunomicrograph adapted from Clark et al.121). [B(i)] The use of CRISPR/cas9 gene editing to generate IPSC-derived nociceptors edited to constituently express HA-epitope tagged Nav1.7. (ii) Current-clamp analysis of the current require to elicit and action potential of IPSC-derived nociceptors derived from healthy participants and congenital insensitivity to pain (CIP, due to Nav1.7 loss-of-function) participants. CIP participant IPSC-derived nociceptors were hypoexcitable. The CIP participant hypoexcitability was corrected using CRISPR/Cas9 IPSC editing. CRISPR/Cas9 was used to generate Nav1.7-KO IPSCs that were differentiated into nociceptors that mimicked CIP hypoexcitability (adapted from McDermott et al.106). (C) Current-clamp analysis of the rheobase (minimum current required to elicit and action potential) of IPSC-derived nociceptors from wild-type participants, and participants carrying the F139WfsX24 TRESK loss-of-function mutation. IPSC-derived nociceptors from F139WfsX24 are hyperexcitable compared to wild-type nociceptors. This nociceptor hyperexcitability was rescued when using CRISPR/cas9 to correct the TRESK mutation (adapted from Pettingill et al.103). (D) IPSC-derived nociceptors transduced with the chemogenetic silencing tool GluCl. Scale bar = 50 µm. Patch clamp analysis revealed that following application of the GluCl agonist ivermectin (IVM), GluCl+ nociceptors were rendered either fully silent or partially silent to depolarizing current injections (adapted from Weir et al.122). (E) Example of stem cell derived nociceptors that have been used to model a chemical injury model through the addition of H2O2. H2O2 treatment leads increased caspase expression and neurite retraction/disassembly (adapted from Jones et al.119) [F(i)] IPSC-derived nociceptors derived from healthy control (HC1) or CRIPSR/cas9 engineered Nav1.7 KO lines. The Nav1.7 blocker BIIB074 promoted action potential failure in both HC1 and Nav1.7 KO lines. (ii) Current clamp recordings of HC1 and Nav1.7 KO nociceptors in the presence of vehicle or PF-05089771. Only HC1 but not Nav1.7 KO nociceptors demonstrated changes in excitability in response to the PF-05089771 compound (adapted from McDermott et al.106). (G) Multi-electrode arrays (MEAs) used to assess spontaneous activity of IPSC-derived nociceptors from an IEM participant. Activity at electrodes was reduced after addition of the TRESK activator cloxyquin. Spontaneous activity recovery was lost following co-addition of TPA (TRESK inhibitor) and cloxyquin (adapted from Pettingill et al.103).