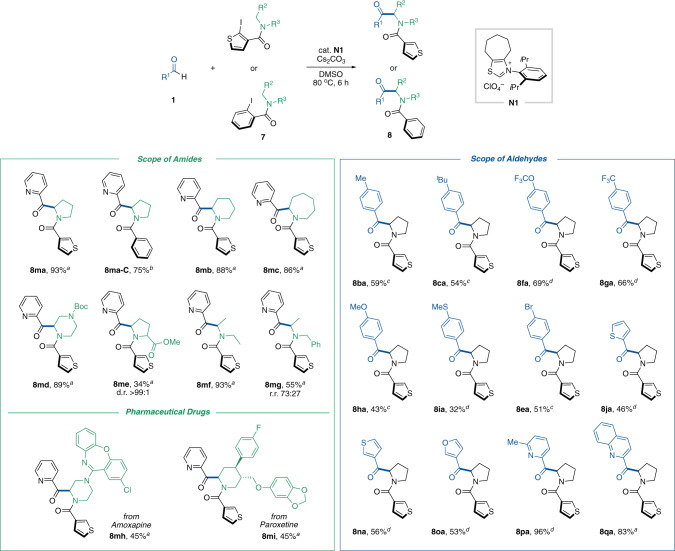
Fig. 4. Substrate scope of C(sp3)–H acylation of secondary amides.
a Reaction was carried out with 1 (0.4 mmol), 2 (0.2 mmol), N1 (10 mol %), and Cs2CO3 (0.22 mmol) in DMSO (0.4 mL) at 80 °C for 6 h. b Reaction was carried out with 1 (0.4 mmol), 2 (0.2 mmol), N1 (5 mol %), and Cs2CO3 (0.21 mmol) in DMSO (0.4 mL) at 80 °C for 6 h. c Reaction was carried out with 1 (0.6 mmol), 2 (0.2 mmol), N1 (20 mol %), and Cs2CO3 (0.24 mmol) in DMSO (0.4 mL) at 80 °C for 6 h. d Reaction was carried out with 1 (0.4 mmol), 2 (0.2 mmol), N1 (20 mol %), and Cs2CO3 (0.24 mmol) in DMSO (0.4 mL) at 80 °C for 6 h.