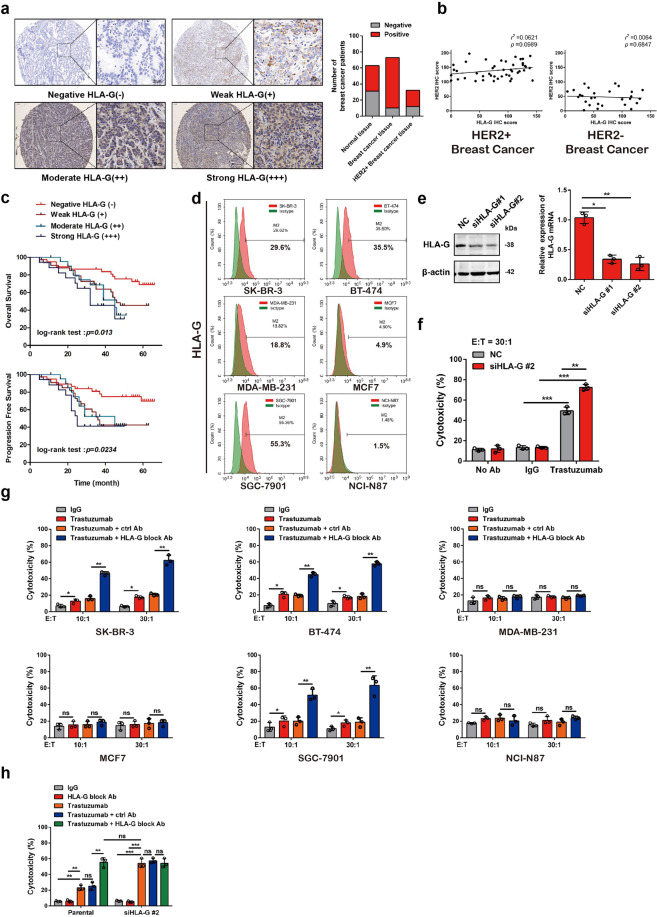
Fig. 1.
HLA-G depletion on HER2-positive breast cancer cells enhances trastuzumab-induced lysis by cocultured NK cells. a Representative immunohistochemical staining of HLA-G in a breast cancer tissue array. (Right panel) The number of HLA-G-positive and -negative normal and neoplastic tissues obtained from patients with HER2-positive or other subtypes of breast cancer were plotted. b Correlation analysis for HLA-G and HER2 expression in breast cancer tissues. c Kaplan–Meier survival analysis based on the prognosis and recurrence rates of 108 HER2-positive breast cancer patients grouped by HLA-G expression. d FCM examination of HLA-G expression in breast cancer and gastric cancer cell lines. e Western blot analysis (left) and qRT-PCR assay (right) of HLA-G expression in SK-BR-3 cells 48 h after transfection with the indicated siRNA. f NK cells prepared from PBMCs of healthy donors (effector, abbreviated as “E”) were cocultured with control or HLA-G siRNA-transfected SK-BR-3 cells (target, abbreviated as “T”) supplemented with or without the indicated antibodies. The cytotoxicity of the NK cells was measured via FCM as described in the “Materials and methods.” g NK cells in the presence of trastuzumab were cocultured with different neoplastic cell lines at the indicated E:T ratios supplemented with or without a control or HLA-G blocking antibody. The cytotoxicity of NK cells was measured via FCM. h Parental or HLA-G knockdown (siHLA-G#2) SK-BR-3 cells were cocultured with NK cells (E:T = 10:1) in the presence of trastuzumab with or without an HLA-G-blocking antibody. The cytotoxicity of NK cells was measured via FCM. All experiments were performed three times. Statistical significance was determined by Student’s t test. *P < 0.05, **P < 0.01, and ***P < 0.001. n.s. Nonsignificant