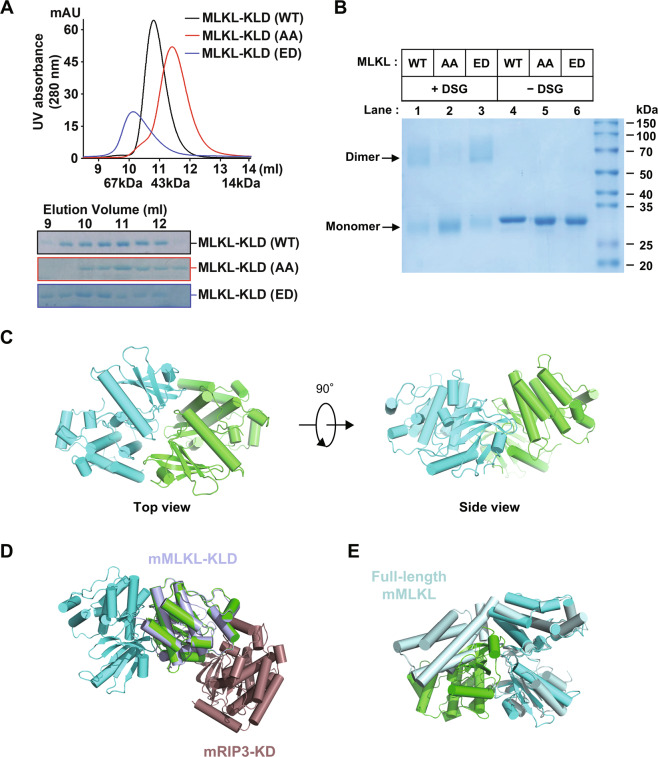
Fig. 1. The dimerization of MLKL kinase-like domain.
A Gel filtration analysis of purified recombinant human MLKL kinase-like domain (KLD) proteins on Superdex 75. The elution fractions were applied to SDS-PAGE followed by Coomassie blue staining. The elution positions of size standards are indicated (BSA, 67 kDa; Ovalbumin, 44 kDa; ribonuclease A, 14 kDa). B Detection of self-assembly of recombinant human MLKL kinase-like domain proteins using chemical crosslinker. Aliquots of purified recombinant wild-type or mutant forms of MLKL KLD proteins were mixed with DMSO or crosslinker DSG as described in the MATERIALS AND METHODS, Samples were subjected to SDS-PAGE and stained by Coomassie blue. Abbreviations are as follows: DSG, disuccinimidyl glutarate; WT, wild-type; AA, phosphor-sites’ mutant, T357A/S358A; ED, phosphomimic mutant, T357E/S358D. C Two perpendicular views of the overall structure of the phosphomimic mutant (T357E/S358D) of human MLKL kinase-like domain homodimer (green and cyan). All structural figures were prepared with PyMOL. D Structural comparison of the phosphomimic mutant (T357E/S358D) of human MLKL kinase-like domain homodimer (green and cyan) with the complex structure of mouse MLKL kinase-like domain (bluewhite) and mouse RIP3 kinase domain (brown) (PDB: 4M69). E Structural comparison of the phosphomimic mutant (T357E/S358D) of human MLKL kinase-like domain homodimer (green and cyan) with the full-length structure of mouse MLKL (palecyan, PDB: 4BTF).