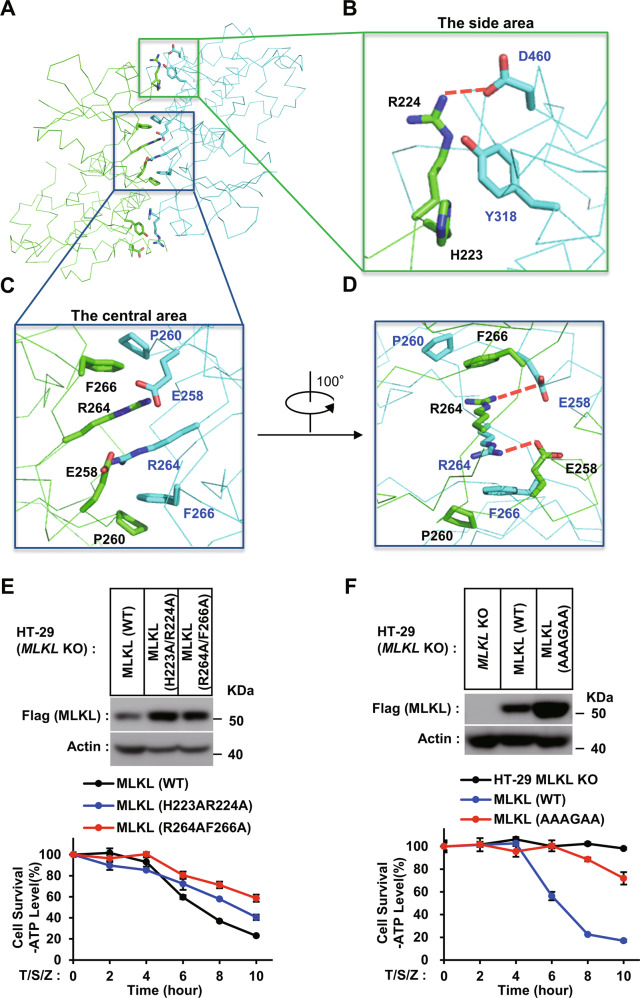
Fig. 2. MLKL kinase-like domain dimerization is required for MLKL-dependent necroptosis.
A Overall structure of MLKL kinase-like domain dimer (green and cyan), illustrating the central and the side of dimer interface. The interface residues involved in intermolecular bonding are shown in sticks. B A close-up view on the side of the dimer interface. Arg224 of one MLKL molecular interacted with Glu460 of the other molecular by ionic bond. The phenyl rings from Tyr318 of one MLKL molecular hydrophobic interacted with the hydrocarbon chain of Arg224 and backbone of His223 of the other molecular. Ionic bond is in this and all other figures are represented by red dashed lines. C, D Close-up views on the center of dimer interface. Glu258 of one MLKL molecule ionically interacted with Arg264 of the other molecule. Pro260 of one MLKL and Phe266 of the other molecular stack against each other by CH/π interaction. E, F The effect of dimerization mutants of MLKL on necrosis. MLKL KO HT-29 cells stably expressing C-terminal flag-tagged wild-type (WT) or dimerization mutants H223A/R224A and R264A/F266A (E) or H223A/R224A/E258A/P260G/R264A/F266A (AAAGAA) (F) of MLKL by lentivirus infection were treated with T/S/Z for the indicated time. The number of surviving cells was determined by measuring ATP levels using the CellTiter-Glo kit (lower panel). The data are presented as the mean ± SD of duplicate wells. Similar results were obtained from at least three independent experiments. Abbreviations are as follows: T, TNF-α; S, Smac mimetic; Z, z-VAD-fmk. The final concentrations of 10 ng/ml TNF-α, 100 nM Smac mimetic, and 20 μM z-VAD-fmk were used. Identical concentrations of these necroptosis-inducing agents were used in subsequent experiments unless otherwise stated. The untreated cells were harvested and whole-cell extracts were prepared and normalized to the same concentration. Aliquots of 20 μg whole-cell lysates were subjected to SDS-PAGE followed by western blot analysis of MLKL(by anti-Flag antibody) and β-Actin which is shown as a loading control (upper panel).