Figure 2.
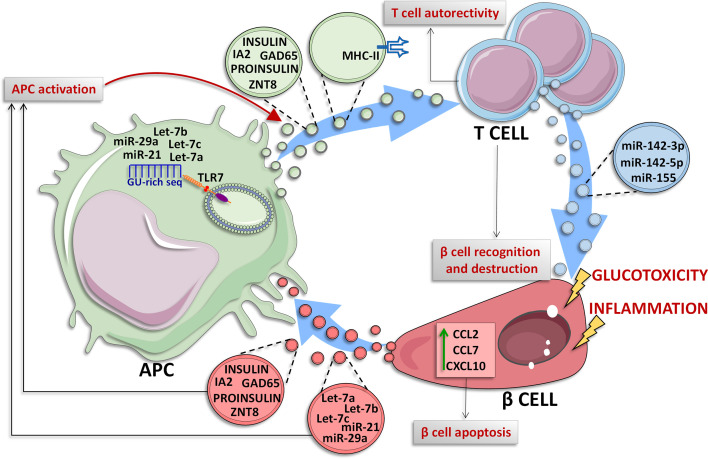
Crosstalk between β cells and immune cells through EVs. In T1D context, β cells and immune cells tightly communicate each other. β cells are subjected to glucotoxic and/or inflammatory stress and can release EVs containing specific miRNAs characterized by a GU-rich sequence (let-7a/b/c, miR-21 and miR-29a) which are transferred to resident Antigen Presenting Cells (APCs), where these miRNAs can bind to endosomal Toll Like Receptor 7 (TLR7) leading to the activation of inflammatory signals (140–142). Moreover, under inflammatory stress, β cells secrete and transfer EVs to APCs. Such EVs can contain specific autoantigens such as insulin, IA2, ZNT8, GAD65 and proinsulin (5) thus leading to their transfer to APC which can present these antigens for adaptive immunity activation. Activated APC can also lead to CD4+ T cells activation through two different mechanisms: (i) release of EVs containing insulin, IA2, ZNT8, GAD65 and proinsulin autoantigens (4), or (ii) release of EVs exposing MHC-II on their surface through which APCs present autoantigens to CD4+ T cells (86, 87), leading to autoreactive T cell activation and subsequent β cell destruction. In T1D context, pancreatic islet-infiltrating T cells secrete a specific subpopulation of EVs carrying miR-142-3p/5p and miR-155 which can be transferred to β cells; these miRNAs cause the upregulation of inflammatory molecules such as CCL2, CCL7 and CXCL10 leading to β cell apoptosis (6).