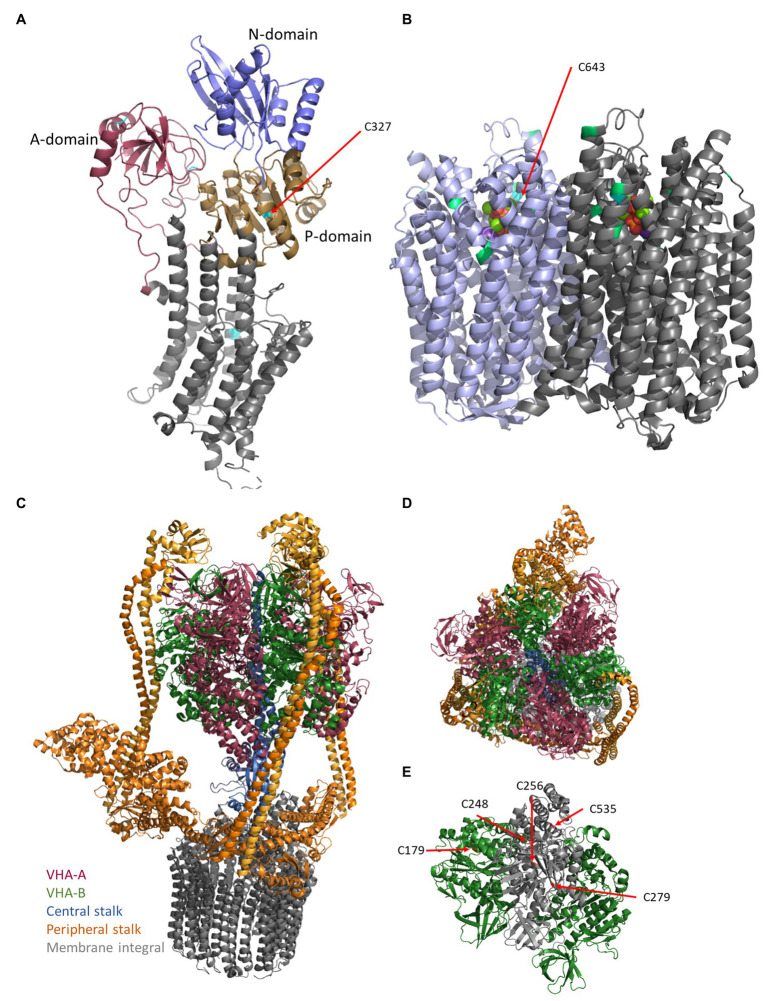
Figure 1.
Structures of the proton pumps. The structures of plasma membrane-ATPase (PM-ATPase) AHA2 of Arabidopsis thaliana (A) and vacuolar pyrophosphatase (V-PPase) from Vigna radiata (B) base on the pdb-files 5KSD (Croll and Andersen, 2016; Focht et al., 2017) and 6AFS (Tsai et al., 2019), respectively. (A) The PM-ATPase consists of 10 transmembrane domains and a large cytoplasmic C-terminal domain (Almeida et al., 2017; Nguyen et al., 2020). In detail, the structure of the cytosolic domain can be divided into the actuator- (A-domain), the nucleotide-binding- (N-domain), which is embedded in the phosphorylation domain (P-domain), and a disordered C-terminal region. ATP binds to the nucleotide-binding domain, which in turn moves closer to the phosphorylation domain, thereby forming the catalytic site. Cysteine residues are indicated by cyan coloring. (B) The V-PPase (EC 3.6.1.1) functions as FIGURE 1homodimeric proton pump of 160 kDa at the tonoplast and acidifies vacuoles, in particular, vacuoles of expanding cells (Smart et al., 1998; Maeshima, 2001). It consists of 16 transmembrane domains, of which six helices are required for proton transport and cytosolic domains form five Mg2+-binding sites in V. radiata (Tsai et al., 2019). The 14-3-3 binding sites (green) and the conserved Cys643 (cyan) are highlighted. One molecule pyrophosphate is bound each monomer and visible as balls in the center. (C) The vacuolar-type ATPase (V-ATPase) consists of the membrane integral sector VO (VHA-a, VHA-c, VHA-c”, VHA-d, and VHA-e) and the membrane associated sector V1 (VHA-A–VHA-H), which represent the proton translocator (gray) and ATPase, respectively (C). Proton transport occurs by rotation of a proteolipid ring formed by multiple copies of VHA-c and a single copy of VHA-c”. VHA-a contributes half channels as proton inlet and outlet pipes. The protons have accessed to a conserved glutamate residue of the proteolipids, protonated the amino acid, and become deprotonated after one approximately one turn by a positive barrier charge of VHA-a, which alters the pKa of the glutamate so that the proton is released into the lumen. VHA-d serves as a bearing between the proteolipid ring and the central stalk of V1. This central stalk (blue) is formed by VHA-D and VHA-F and transduces the sequence of conformational alterations due to ATP-hydrolysis within three copies of VHA-A into rotation. (C,D) VHA-A (red) and VHA-B (green) form a hexameric head around (red/green) the central stalk (blue) and are anchored to the membrane by three rigid peripheral stalks (each formed by VHA-E and VHA-G heterodimer, orange), which are crosslinked by VHA-C (orange) and VHA-H (orange) and anchored to the membrane via a cytosolic domain of VHA-a, so that VHA-a is essential for proton transport and avoids co-rotation of the head structure. (E) Within VHA-A, Cys256, Cys279, and Cys535 are highly conserved among all eukaryotes, Cys248 is plant-specific and the distance between Cys Cys256 and Cys248 is approximately 11 Å and would allow for disulfide formation. VHA-B bears Cys179, which is target of redox modification. The pdb-file 3j9t was used as template (Zhao et al., 2015).