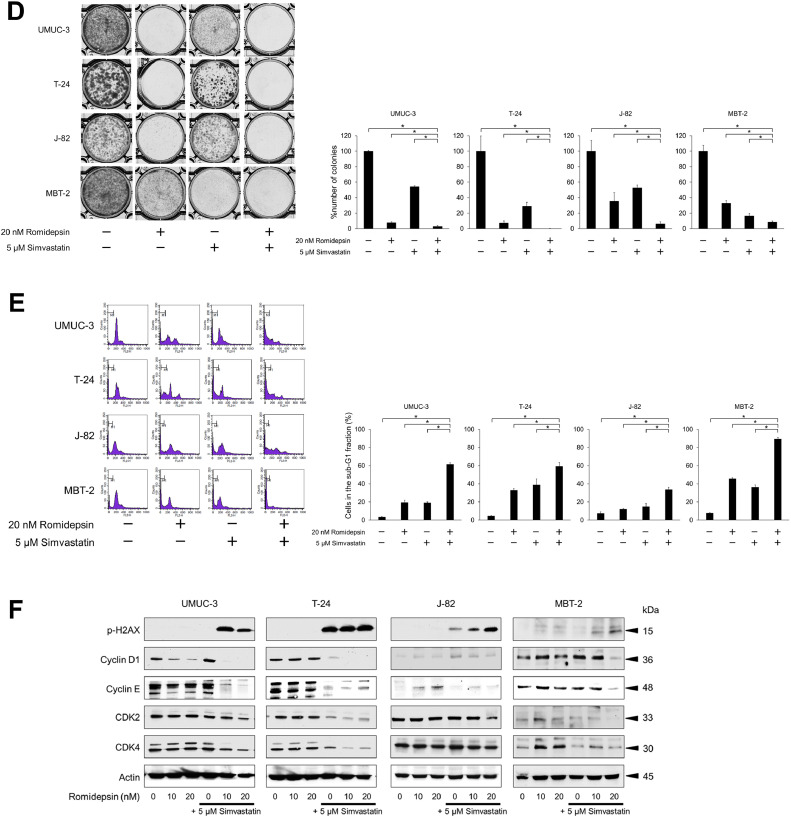
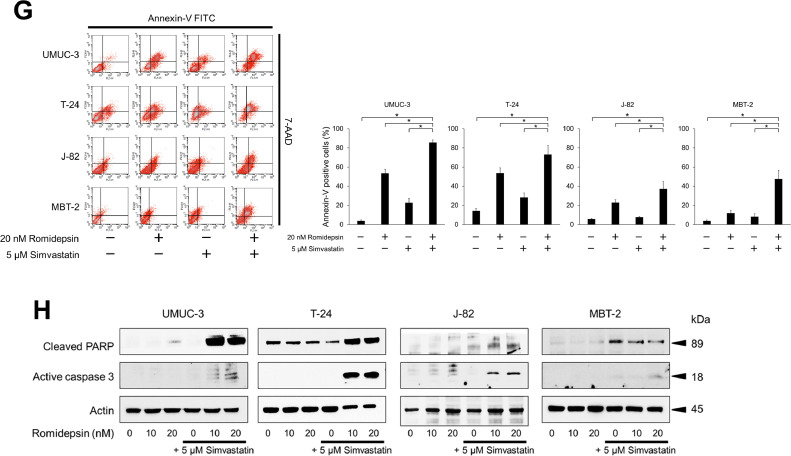
Fig. 2.
Anticancer activity of the simvastatin-romidepsin combination in bladder cancer cells. (A) Cells were treated for 48 h with 2.5–5 μM simvastatin and/or 5–20 nM romidepsin and cell viability was measured using CCK-8 assay. Bars represent mean ± SD, n = 6. (B) Photomicrographs showing morphological changes of the cells after 48-hour treatment with 5 μM simvastatin and/or 20 nM romidepsin. Scale bar = 300 μm. (C) Cells were given 5 μM simvastatin and/or 20 nM romidepsin and confluence measurements were performed at 3-hour intervals over 3 days. Mean ± SD, n = 6. (D) Clonogenic assay. 200–300 cells were treated for 48 h with 5 μM simvastatin and/or 20 nM romidepsin. The cells were then given fresh media and incubated for 1–2 weeks. Bar graphs show the%number of colonies relative to the untreated control. Mean ± SD, n = 3. *p = 0.0495. (E) Cells were treated for 48 h with 5 μM simvastatin and/or 20 nM romidepsin. Changes in the cell cycle were evaluated using flow cytometry. 10,000 cells were counted. Bar graphs show the percentages of the cells in the sub-G1 fraction. Data are expressed as mean ± SD from three independent experiments. *p = 0.0495. (F) Western blotting for phosphorylated histone H2AX (p-H2AX), cyclin D1, cyclin E, cyclin-dependent kinase (CDK) 2, and CDK4. Cells were treated with 5 μM simvastatin and/or 10–20 nM romidepsin for 48 h. Actin was used for the loading control. Representative blots are shown. (G) Cells were treated for 48 h with 5 μM simvastatin and/or 20 nM romidepsin. Apoptotic cells were detected by annexin-V assay using flow cytometry. 10,000 cells were counted. Bar graphs show the percentages of apoptotic cells. Data are expressed as mean ± SD from three independent experiments. FITC, fluorescein isothiocyanate; 7-AAD, 7-amino-actinomycin D. *p = 0.0495. (H) Western blotting for cleaved poly(ADP-ribose) polymerase (PARP) and active caspase 3. Cells were treated with 5 μM simvastatin and/or 10–20 nM romidepsin for 48 h. Actin was used for the loading control. Representative blots are shown. (I) Western blotting for AMP-activated protein kinase (AMPK), acetylated histone, and peroxisome proliferator-activated receptor (PPAR) γ. Cells were treated with 5 μM simvastatin and/or 10–20 nM romidepsin for 48 h. Actin was used for the loading control. Representative blots are shown. (J) Western blotting for glucose-regulated protein (GRP) 78, endoplasmic reticulum resident protein (ERp) 44, histone deacetylase (HDAC) 1, HDAC3, HDAC6, and acetylated α-tubulin. Cells were treated with 5 μM simvastatin and/or 10–20 nM romidepsin for 48 h. Actin was used for the loading control. Representative blots are shown. (K) Cells were treated with 5 μM simvastatin and/or 20 nM romidepsin for 48 h and reactive oxygen species production was measured by dihydroethidium (DHE) staining using flow cytometry. 10,000 cells were counted. Bar graphs show the relative DHE fluorescence intensity. Data are expressed as mean ± SD from three independent experiments. *p = 0.0495.