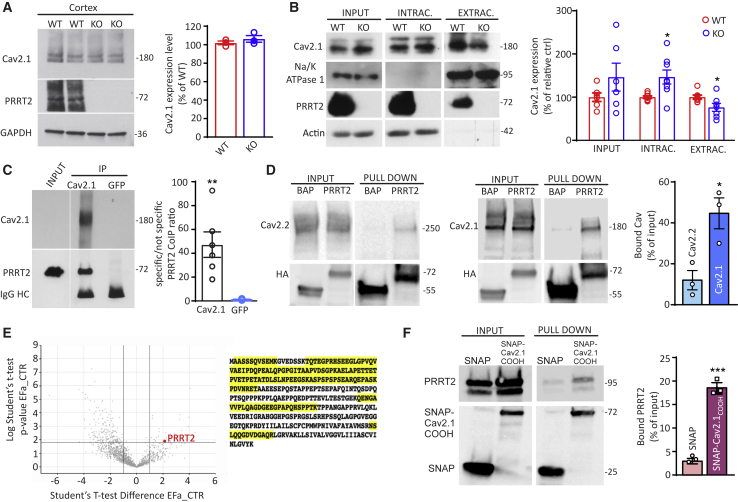
Figure 5.
PRRT2 directly interacts with P/Q-type Ca2+ channels
(A) Left: representative immunoblots of the expression levels of Cav2.1 in total cortical lysates of WT and PRRT2 KO mice. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) immunoreactivity was included as a control of equal loading. Right: quantification of Cav2.1 expression in WT and PRRT2 KO mice normalized on GAPDH expression and expressed in percentage of the mean WT immunoreactivity (means ± SEMs with superimposed individual values; n = 3 independent preparations).
(B) Representative immunoblots of cell surface biotinylation performed in primary hippocampal WT and PRRT2 KO neurons (left panel). Total cell lysates (input), biotinylated (extracellular), and non-biotinylated (intracellular) fractions were analyzed by immunoblotting. Na/K-ATPase 1 and actin were included as markers of plasma membrane and cytosolic fractions, respectively. Individual values and means ± SEMs of total cell, extracellular, and intracellular Cav2.1 expression normalized on Na/K-ATPase 1 and actin expression (n = 5 independent preparations).
(C) Co-immunoprecipitation of PRRT2 with Cav2.1. Detergent extracts of mouse brain were immunoprecipitated (IP) with Cav2.1 antibodies or with an anti-GFP antibody, as indicated. After electrophoretic separation of the immunocomplexes and western blotting, membranes were probed with anti-Cav2.1 antibodies to test the immunoprecipitation efficiency, as well as with anti-PRRT2 antibodies to probe the interaction. Left: a representative immunoblot is shown. Right: quantification of the PRRT2 immunoreactive signal in the IP samples, normalized to the binding of the anti-GFP control (means ± SEMs with superimposed individual values, n = 6 independent experiments). Immunoglobulin G (IgG) HC, antibody heavy chain. Input, 10 μg total extract.
(D) Specificity of PRRT2 for Cav2.1 over Cav2.2. Extracts of HEK293 cells transiently expressing HA-tagged PRRT2 and either Cav2.1 or Cav2.2 were subjected to affinity precipitation with anti-HA agarose beads. Left panel: representative immunoblot showing input and pulled-down fractions from extracts of Cav2.2-expressing HEK293 cells. Center panel: representative immunoblot showing input and pulled-down fractions from extracts of Cav2.1-expressing HEK293 cells. VGCC (top) and PRRT2-HA (bottom) immunoblots are shown. Right panel: quantitative evaluation of the amounts of Cav2.1 and Cav2.2 immunoreactivities bound to PRRT2-HA, expressed in percentage of the respective input (means ± SEMs with superimposed individual values, n = 3 independent experiments).
(E) Proteomic screen using the C-terminal domain of the Cav2.1 as a bait. Left: volcano plot combining fold change (FC in log2) with statistical significance (p value in l-log10). PRRT2 is purified with FC > 4 versus control with a corresponding p = 0.012. Right: primary amino acid sequence of mouse PRRT2 with the exclusive spectra sequences identified by mass spectrometry analysis highlighted in yellow.
(F) Pull down of PRRT2 by the C-terminal domain of Cav2.1. A SNAP fusion protein of the C-terminal domain of Cav2.1 was used as a bait for pulling down PRRT2 from an extract of PRRT2-expressing HEK293 cells. Pull down with SNAP alone was used as an internal control. Left: a representative immunoblot is shown. Right: quantification of the PRRT2 immunoreactive signal in the pulled-down samples, expressed in percentage of the respective input (means ± SEMs with superimposed individual values, n = 3 independent experiments). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, unpaired Student’s t test/Mann-Whitney U test.