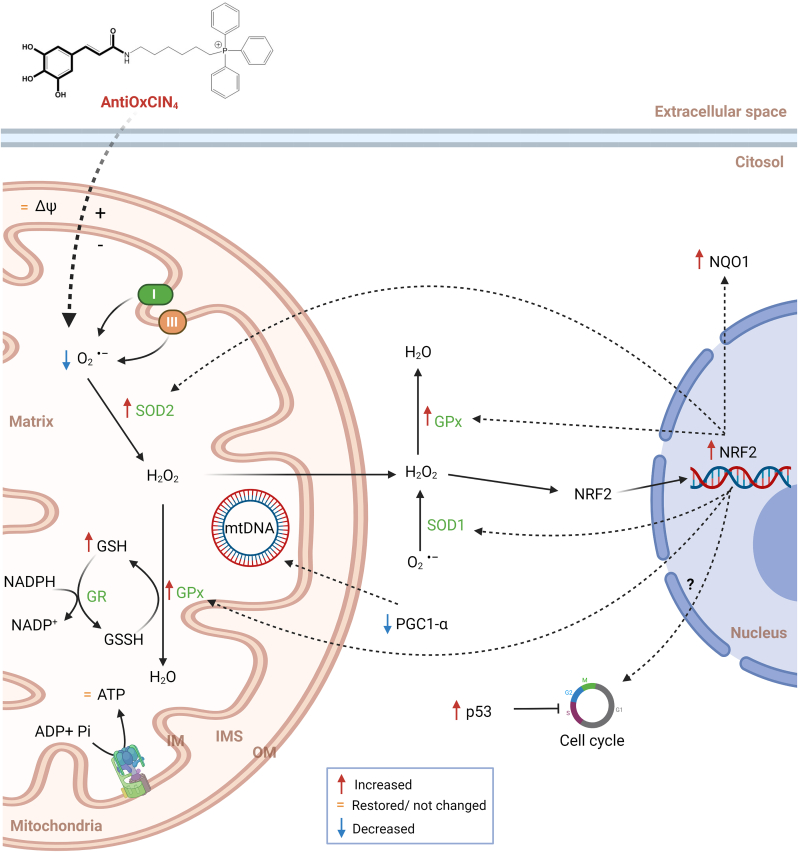
Fig. 13.
Schematic representation of the mechanisms underlying AntiOxCIN4 beneficial effects in fibroblasts from sPD patients. A new dietary antioxidant based on caffeic acid scaffold was conjugated with TPP cation (AntiOxCIN4), allowing its preferential accumulation into mitochondria. Treatment of fibroblasts from sPD patients with non-lethal AntiOxCIN4 concentrations restores mitochondrial membrane potential, decreases ROS levels and stimulates gene expression of the Nuclear factor erythroid 2-related factor 2 (NRF2). The latter stimulates the total activity of superoxide dismutases (SOD), thereby facilitating the conversion of mitochondrial superoxide (O2•-) into hydrogen peroxide (H2O2) and inhibiting ROS-induced cell death. Downstream of SOD action, H2O2 is converted into H2O by glutathione peroxidases (GPx) in a glutathione (GSH)-dependent manner. Oxidized GSH (GSSG) is regenerated to GSH by the action of glutathione reductase (GR), using electrons supplied by NADPH. AntiOxCIN4 increased the gene expression of the NAD(P)H dehydrogenase (quinone) 1 (NQO1), which is a NRF2 target. Due to low ATP levels of fibroblasts from sPD patients treated with AntiOxCIN4, AMPK promotes S-phase cell cycle arrest in a p53-dependent manner to restore normal mitochondrial function and avoid a deleterious mitochondrial phenotype. Activation of NRF2 can also result in cell cycle arrest at an early stage of oxidative stress response mechanisms. Downregulation of mitochondrial biogenesis and autophagic flux occurred as a compensatory mechanism and restored mitochondrial fission by decreasing mitochondrial elongation, most likely by decreasing DRP1 phosphorylation at ser673. A general improvement in mitochondrial health in fibroblasts from sPD patients is exhibited by restored maximal respiration, decreased mitochondrial swelling, and increased cellular metabolic activity (further details are provided in the Discussion).