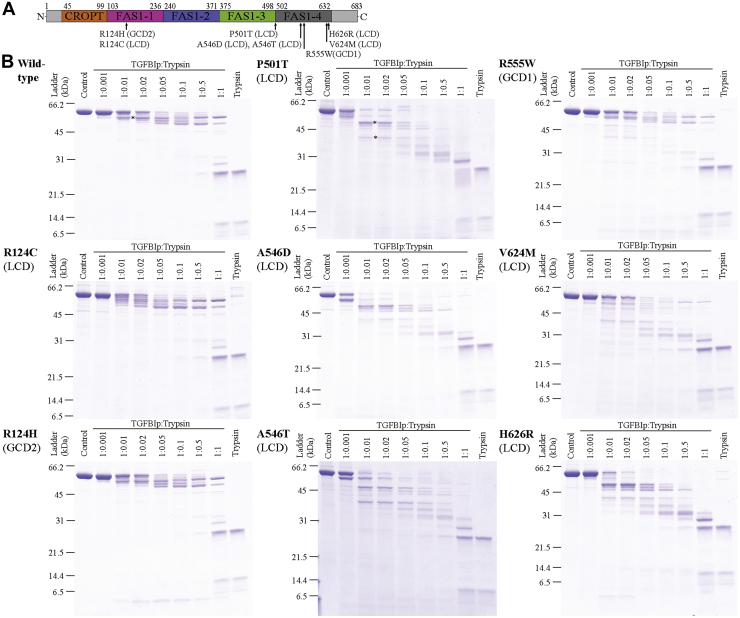
Figure 1.
LCD mutations induce changes in the proteolytic stability of TGFBIp.A, domain organization of TGFBIp indicating the location of the mutated residues. B, limited proteolysis of wildtype TGFBIp and eight mutants; 0.8 μM of TGFBIp wildtype and mutants was incubated with a titration series of trypsin for 90 min at 37 °C and subsequently analyzed by reducing SDS-PAGE. TGFBIp to trypsin ratios (w/w) are depicted at the top of the gels. The asterisks (∗) mark cleavage products with an intact N terminus as determined by Edman degradation. All the mutants associated with LCD, except R124C, were more readily degraded by trypsin than wildtype TGFBIp, whereas R555W and R124H associated with GCD were as stable as wildtype TGFBIp.