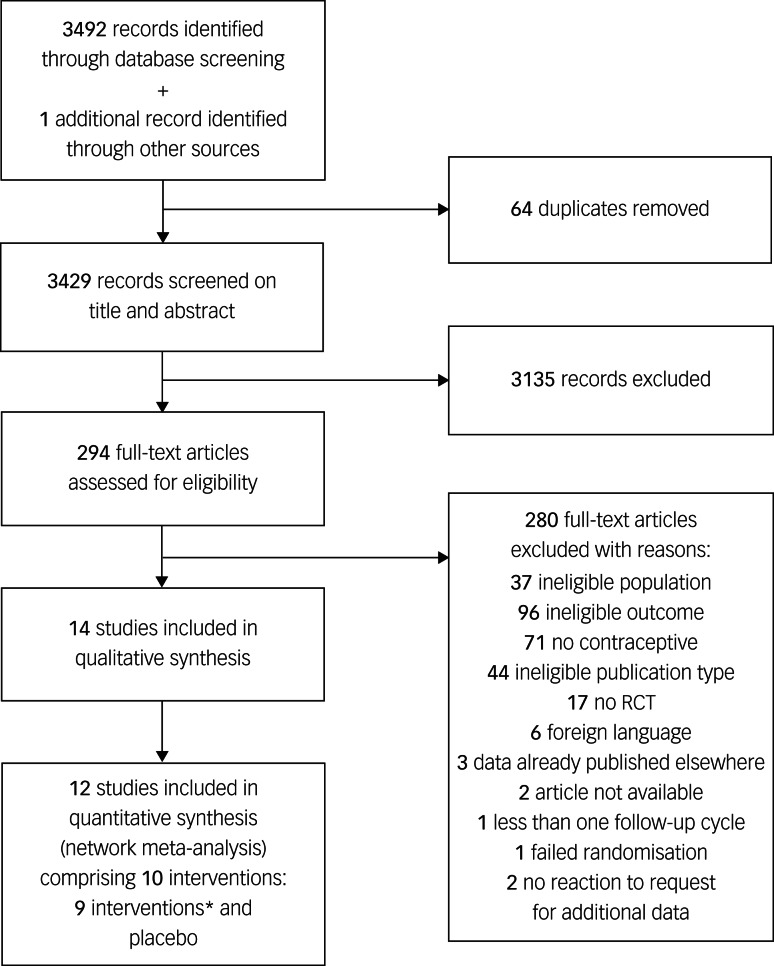
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
*Ethinylestradiol/drospirenone (30 μg/3 mg), ethinylestradiol/etonogestrel (15 μg/120 μg), oestradiol/dienogest (3;2;2;1 mg/0;2;3;0 mg), desogestrel (75 μg), ethinylestradiol/levonorgestrel (30 μg/150 μg), levonorgestrel (30 μg), oestradiol/nomegestrol acetate (1.5 mg/2.5 mg), ethinylestradiol/levonorgestrel (20 μg/100 μg) and ethinylestradiol/desogestrel (20 μg/150 μg). RCT, randomised clinical trial.