Abstract
Aims: To investigate the effects of single nucleotide polymorphisms (SNPs) in genes of one-carbon metabolism (OCM) related enzymes and anti-epileptic drug (AED) monotherapy on homocysteine (Hcy) metabolism in patients with epilepsy, and to further explore specific SNPs that may increase patients' susceptibility to the effects of AEDs on the Hcy imbalance.
Method: This case-control study analyzed 279 patients with epilepsy, including patients receiving monotherapy with valproate (VPA) (n = 53), oxcarbazepine (OXC) (n = 71), lamotrigine (LTG) (n = 55), or levetiracetam (LEV) (n = 35) and patients who had not taken any AEDs (controls, n = 65) for at least 6 months. Serum levels of vitamin B12 (vit B12), folate (FA) and Hcy were measured, and 23 SNPs in 13 genes of OCM-related enzymes were genotyped in all patients.
Results: Methylenetetrahydrofolate reductase (MTHFR) rs1801133 was associated with elevated serum Hcy levels in patients with epilepsy (P < 0.001), and patients presenting the TT genotype exhibited higher serum Hcy levels than patients with the CC (P < 0.001) or CT (P < 0.001) genotype. A subsequent multiple linear regression analysis showed that AED monotherapy with VPA (vs. control: P = 0.023) or OXC (vs. control: P = 0.041), and genotypes of MTHFR rs1801133 TT (vs. CC: P < 0.001; vs. CT: P < 0.001), transcobalamin 2 (TCN2) rs1801198 CC (vs. GC: P = 0.039) and folate receptor 1 (FOLR1) rs2071010 AA (vs. GA: P = 0.031) were independent risk factors for higher Hcy levels. In the subgroup analysis of patients taking OXC, we found that patients with genotypes of MTHFR rs1801133 TT (vs. CC: P = 0.001; vs. CT: P < 0.001) and TCN2 rs1801198 CC (vs. GC: P = 0.021; vs. GG: P = 0.018) exhibited higher serum Hcy levels.
Conclusions: VPA, OXC, and genotypes of MTHFR rs1801133 TT, TCN2 rs1801198 CC, and FOLR1 rs2071010 AA are all independent risk factors for elevated Hcy levels in patients with epilepsy. Moreover, genotypes of MTHFR rs1801133 TT and TCN2 rs1801198 CC may increase patients' susceptibility to the effect of OXC on disrupting Hcy homeostasis.
Keywords: epilepsy, single nucleotide polymorphism, anti-epileptic drug, homocysteine, one-carbon metabolism
Introduction
Previously published data show that patients with epilepsy on chronic anti-epileptic drug (AED) therapy are more susceptible to hyperhomocysteinemia than the general population (1–4). Hyperhomocysteinemia is a dominant probable risk factor for various medical conditions, such as cardiovascular disease (5, 6), osteoporosis (7–9), stroke (10–12), neurodegenerative diseases (13, 14) and neural tube defects (NTDs) (15–17). Moreover, hyperhomocysteinemia may enhance seizure activity and lead to antiepileptic drug resistance as shown in animal model experiments (18–20).
Two pathways are available for the removal of homocysteine (Hcy): transsulfuration and remethylation. In the former pathway, Hcy is catalyzed by cystathionine synthase (CBS) in the presence of serine to form cystathionine, a vitamin B6–dependent reaction. In the latter pathway, both 5-methyltetrahydrofolate (5-mTHF) and betaine can act as methyl donors for the remethylation of Hcy through folate- and betaine-dependent pathways, respectively (21). 5,10-methylenetetrahydrofolate reductase (MTHFR), a key regulatory enzyme, plays an important role in Hcy homeostasis by catalyzing the conversion of 5,10-methylenetetrahydrofolate (5,10-CH2-THF) to 5-mTHF, which is catalyzed by methionine synthesis using vitamin B12 as a cofactor for the remethylation of Hcy to methionine (22). Betaine homocysteine methyltransferase (BHMT), which is expressed at high levels in the human liver, also helps maintain the Hcy balance by catalyzing the transfer of a methyl group from betaine to Hcy to generate methionine (23). Then methionine is activated through the action of methionine adenosyltransferase (MAT) to produce s-adenosylmethionine (SAM) which is the ubiquitous methyl donor in a vast array of intracellular transmethylation reactions. Afterwards, s-adenosylhomocysteine (SAH), the end product of all SAM-dependent transmethylation reactions, is rapidly metabolized by SAH hydrolase to produce homocysteine (24). This pathway is the only one that produces Hcy.
As vitamin B12 and folate are essential cofactors for the remethylation of Hcy, decreased blood levels of these nutrients disrupt Hcy metabolism and lead to hyperhomocysteinemia (25). AEDs are believed to interfere with Hcy homeostasis, at least in part, by disturbing the intestinal absorption of folate (FA), influencing CYP450 enzymatic reactions and the subsequent consumption of FA, or changing one-carbon metabolism (OCM)-related enzyme activity (1, 26–28). Phenytoin and carbamazepine are mostly likely to increase homocysteine levels. In recent years, phenytoin is rarely used as monotherapy in the treatment of epilepsy, and carbamazepine has to some extent been replaced by oxcarbazepine in clinical practice (29). However, effects of the second generation AEDs on Hcy metabolism are still waiting to be clarified. Thus, four most commonly used antiepileptic drugs in monotherapy [e.g., valproate (VPA), oxcarbazepine (OXC), lamotrigine (LTG), and levetiracetam (LEV)] were finally included in this study. In addition to the aforementioned environmental factors, genetic factors may also disturb Hcy homeostasis. Single nucleotide polymorphisms (SNPs) in genes involved in the OCM pathway increase blood Hcy levels by changing enzyme activity. The genotypes of MTHFR rs1801133 TT and transcobalamin 2 (TCN2) rs1801198 GG were reported to be associated with higher Hcy levels (27, 30, 31). A genetic polymorphism in methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) rs2236225 was also discovered to increase Hcy levels (32).
However, to date, the effects of genetic polymorphisms in the OCM pathway on blood Hcy levels in patients with epilepsy receiving the most commonly used AED monotherapy are unclear. Thus, we conducted this case-control study to investigate the effects of SNPs in genes encoding OCM-related enzymes and AEDs (e.g., VPA, OXC, LTG or LEV monotherapy) on Hcy levels in patients with epilepsy and to further explore specific SNPs that may increase patients' susceptibility to the effects of AEDs on Hcy levels.
Materials and Methods
Subjects
From May 2013 to October 2019, patients with epilepsy (aged between 15 and 55 years) who were treated with VPA (n = 53), OXC (n = 71), LTG (n = 55), or LEV (n = 35) monotherapy for at least 6 months, were included in this study. Patients with epilepsy who were not treated with any AED for at least 6 months were enrolled as controls (n = 65). Epilepsy caused by ischemic stroke or coexisting with cardiac or peripheral vascular diseases, hematological diseases, tumors, liver or renal diseases constituted criteria resulting in exclusion from the study. All subjects who regularly consumed vitamins or any other drugs, other than AEDs (i.e., levodopa, fibrates, niacin, statins, metformin, methotrexate, sulfasalazine, and so on), known to affect plasma levels of FA or Hcy were also excluded. Patients in the two groups were from the same geographic area and were matched for age, sex and ethnic background. The current study was approved by the Human Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University, and written informed consent was obtained from each participant.
Biochemical Analyses
Approximately 2 ml of venous blood was collected from participants in the sitting position. Serum was quickly separated by centrifugation and stored at −80°C until assayed. The serum levels of vitamin B12, FA and Hcy were detected using an Immulite 2000 autoanalyzer and suitable kits (DPC Diagnostic Products Corporation, Los Angeles, CA, USA) according to the manufacturer's instructions.
Genetic Analyses
Whole-genome DNA was extracted from peripheral blood cells using a TIANamp Blood DNA Kit (Qiagen, Beijing, China) according to the manufacturer's instructions. The DNA concentration and purity were determined by measuring the absorbance at 260 and 280 nm using a NanoDropTM 1,000 Spectrophotometer (Thermo Scientific, Wilmington, USA). Twenty-three SNPs in 13 genes of the OCM pathway were detected by an iPLEX® mass spectrometry-based multiplex genotyping assay (Sequenom, CA, USA), including GCPII rs202676, FOLR1 rs2071010, FOLR2 rs2298444, SLC19A1 rs1051266/rs914238, DHFR rs380691, MTHFD1 rs1950902/rs2236225, MTHFR rs1801131/rs1801133, TCN2 rs1801198, MTRR rs1801394, BHMT rs3733890, DNMT1 rs2114724/rs2241531/rs7253062, DNMT3a rs13036246/rs34048824/rs6722613/rs7575625/rs7587636, and DNMT3b rs2424908/rs6141813, according to a previously described method (33). In Supplementary Material listed 23 candidate SNPs and its PCR primer and extension primer. MassARRAY Typer 4.0 software was used for proper data acquisition and analysis. Assays with a <80% call rate within the same SpectroCHIP were considered as having failed.
Statistical Analysis
SPSS 20.0 for Windows and Prism 8.0.1 were used for statistical analyses. Analyses of parametric variables were performed using either student's t-test or one-way analysis of variance (ANOVA) with a post hoc Bonferroni test, and the results are described as the means ± standard deviations (x ± s). In analyses of non-parametric variables, either the Mann-Whitney U-test or Kruskal-Wallis test with post hoc Mann-Whitney U-test was used. Furthermore, a multiple linear regression analysis was employed to analyze the factors influencing serum Hcy levels. A P-value < 0.05 was considered statistically significant.
Results
Demographic Data
All patients in treatment groups had daily dosages within therapeutic range. Table 1 shows the main characteristics of the study population. No differences in age or sex distribution were observed among the groups. The serum levels of FA and vitamin B12 were significantly different among the five groups (P = 0.004 and P < 0.001, respectively) (Table 1).
Table 1.
Demographic features of the subjects.
| Control | VPA | OXC | LTG | LEV | P-value | |
|---|---|---|---|---|---|---|
| N | 65 (M: 31) | 53 (M: 31) | 71 (M: 35) | 55 (M: 19) | 35 (M: 17) | 0.173 |
| Age, Y | 28.58 ± 10 | 25.81 ± 10.15 | 26.15 ± 8.84 | 27.35 ± 6.57 | 26.26 ± 8.67 | 0.426 |
| FA, nmol/l | 23.38 ± 9.95 | 28.2 ± 12.1 | 21.09 ± 8.18 | 25.86 ± 11.74 | 23.15 ± 11.59 | 0.004 |
| vit B12, pmol/l | 368.82 ± 169.9 | 460.47 ± 185.85 | 307.93 ± 134.65 | 361.84 ± 143.62 | 327.75 ± 146.63 | <0.001 |
| Hcy, μmol/l | 12.06 ± 3.55 | 12.84 ± 5.59 | 14.86 ± 8.35 | 12.42 ± 5.4 | 14.14 ± 7.13 | 0.063 |
Data are presented as means ± SD. The mean serum levels of FA, vit B12 and Hcy among groups were compared using ANOVA. P < 0.05 was considered statistically significant.
VPA, valproate; OXC, oxcarbazepine; LTG, lamotrigine; LEV, levetiracetam; N, number; FA, folate; vit B12, vitamin B12; Hcy, homocysteine.
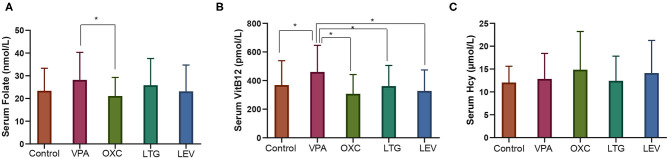
Patients on VPA monotherapy exhibited higher FA levels than patients on OXC monotherapy (28.2 ± 12.1 vs. 21.09 ± 8.18 nmol/l; P = 0.003) (Figure 1A) and higher vitamin B12 levels than patients in the non-AED (460.47 ± 185.85 vs. 368.82 ± 169.9 pmol/l; P = 0.018), LTG (vs. 361.84 ± 143.62 pmol/l; P = 0.012), LEV (vs. 327.75 ± 146.63 pmol/l; P = 0.001) and OXC monotherapy groups (vs. 307.93 ± 134.65 pmol/l; P < 0.001) (Figure 1B). However, the differences in serum Hcy levels among groups were not statistically significant (P > 0.05) (Figure 1C).
Figure 1.
Serum levels of one-carbon metabolites among groups of patients with epilepsy. (A) Serum FA levels, (B) serum VitB12 levels, and (C) serum Hcy levels. *P < 0.05. VPA, valproate; OXC, oxcarbazepine; LTG, lamotrigine; LEV, levetiracetam; N, number; vit B12, vitamin B12; Hcy, homocysteine.
Effects of SNPs in Genes Encoding OCM-Related Enzymes on Hcy Levels in Patients With Epilepsy
The associations between genetic polymorphisms in OCM-related enzymes and serum levels of Hcy in patients with epilepsy were analyzed. In this study, we observed that MTHFR rs1801133 was associated with serum Hcy levels in patients with epilepsy (CC vs. CT vs. TT: 12.4 ± 5.01 vs. 13.44 ± 4.79 vs. 19.92 ± 15.39 μmol/l, P < 0.001), with the TT genotype resulting in higher serum Hcy levels than the CC (P < 0.001) or CT (P < 0.001) genotype (Table 2).
Table 2.
Effects of SNPs in genes encoding OCM-related enzymes on Hcy levels in patients with epilepsy.
| Gene | SNPs | Serum Hcy (μmol/l) | P-value | ||
|---|---|---|---|---|---|
| wild type | Heterozygotes | Homozygotes | |||
| GCPII | rs202676 | 13.18 ± 5.67 (AA: n = 137) | 13.15 ± 6.79 (AG: n = 116) | 13.17 ± 4.52 (GG: n = 24) | 0.999 |
| FOLR1 | rs2071010 | 13.69 ± 7.13 (GG: n = 188) | 12.1 ± 3.47 (GA: n = 83) | 15.04 ± 6.42 (AA: n = 8) | 0.112 |
| FOLR2 | rs2298444 | 13.91 ± 7.46 (TT: n = 105) | 12.93 ± 5.80 (TC: n = 129) | 12.67 ± 4.26 (CC: n = 45) | 0.392 |
| SLC19A1 | rs1051266 | 13.06 ± 5.67 (TT: n = 80) | 13.53 ± 5.69 (TC: n = 129) | 12.7 ± 8.18 (CC: n = 62) | 0.676 |
| rs914238 | 13.13 ± 6.73 (TT: n = 89) | 13.55 ± 6.26 (TC: n = 137) | 12.64 ± 5.62 (CC: n = 51) | 0.665 | |
| DHFR | rs380691 | 13.26 ± 6.22 (GG: n = 99) | 13.48 ± 6.53 (GA: n = 127) | 12.75 ± 5.95 (AA: n = 51) | 0.786 |
| MTHFD1 | rs1950902 | 13.17 ± 6.89 (GG: n = 115) | 13.36 ± 5.72 (GA: n = 125) | 13.12 ± 6.36 (AA: n = 38) | 0.965 |
| rs2236225 | 13.26 ± 6.85 (GG: n = 166) | 13.22 ± 5.31 (GA: n = 100) | 13.24 ± 6.5 (AA: n = 10) | 0.999 | |
| MTHFR | rs1801131 | 13.75 ± 6.87 (TT: n = 159) | 12.88 ± 5.6 (TG: n = 101) | 11.48 ± 3.94 (GG: n = 15) | 0.289 |
| rs1801133 | 12.4 ± 5.01 (CC: n = 156)b | 13.44 ± 4.79 (CT: n = 106)c | 19.92 ± 15.39 (TT: n = 17) | <0.001a | |
| TCN2 | rs1801198 | 13.37 ± 6.83 (GG: n = 95) | 12.63 ± 4.21 (GC: n = 131) | 14.53 ± 8.97 (CC: n = 52) | 0.177 |
| MTRR | rs1801394 | 12.88 ± 6.4 (AA: n = 147) | 13.63 ± 6.21 (AG: n = 103) | 14.62 ± 7.05 (GG: n = 19) | 0.425 |
| BHMT | rs3733890 | 12.59 ± 6.21 (GG: n = 131) | 13.78 ± 6.61 (GA: n = 118) | 13.80 ± 4.81 (AA: n = 26) | 0.29 |
| DNMT1 | rs2114724 | 13.6 ± 6.49 (CC: n = 143) | 12.93 ± 6.49 (CT: n = 107) | 12.65 ± 4.17 (TT: n = 28) | 0.615 |
| rs2241531 | 12.92 ± 6.22 (CC: n = 83) | 12.87 ± 5.18 (CG: n = 139) | 14.79 ± 8.52 (GG: n = 55) | 0.134 | |
| rs7253062 | 13.67 ± 6.77 (GG: n = 143) | 12.84 ± 5.84 (GA: n = 119) | 13.2 ± 5.21 (AA: n = 14) | 0.576 | |
| DNMT3a | rs13036246 | 13 ± 5.44 (CC: n = 143) | 14.02 ± 7.54 (CT: n = 110) | 11.5 ± 4.12 (TT: n = 25) | 0.149 |
| rs34048824 | 13.07 ± 5.65 (TT: n = 168) | 13.83 ± 7.46 (TC: n = 97) | 11.54 ± 4.1 (CC: n = 14) | 0.368 | |
| rs6722613 | 13.14 ± 5.71 (GG: n = 113) | 13.51 ± 6.8 (GA: n = 128) | 12.79 ± 6.24 (AA: n = 37) | 0.796 | |
| rs7575625 | 13.12 ± 5.72 (AA: n = 160) | 13.6 ± 7.25 (AG: n = 106) | 12.09 ± 4.02 (GG: n = 13) | 0.662 | |
| rs7587636 | 13.14 ± 5.78 (GG: n = 119) | 13.62 ± 7.02 (GA: n = 133) | 11.98 ± 4.16 (AA: n = 27) | 0.452 | |
| DNMT3b | rs2424908 | 12.88 ± 4.83 (TT: n = 96) | 13.45 ± 6.61 (TC: n = 138) | 13.46 ± 7.89 (CC: n = 45) | 0.776 |
| rs6141813 | 13.17 ± 5.7 (AA: n = 116) | 13.15 ± 5.99 (AG: n = 130) | 14.11 ± 9.07 (GG: n = 32) | 0.722 | |
Serum Hcy levels were significantly different among groups.
TT vs. CC: 19.92 ± 15.39 vs. 12.4 ± 5.01 μmol/l, P < 0.001.
TT vs. CT: 19.92 ± 15.39 vs. 13.44 ± 4.79 μmol/l, P < 0.001.
P-values were calculated using ANOVA.
SNPs, some single nucleotide polymorphisms; Hcy, homocysteine.
Effects of AEDs and SNPs in OCM Related Enzymes on Serum Hcy Levels in Patients With Epilepsy
We employed multiple linear regression analysis to further explore the effects of AEDs and SNPs in OCM-related enzymes on serum Hcy levels. After adjusting for other factors included in the model, the analysis showed that serum levels of FA and vitamin B12 were negatively associated with serum Hcy levels (FA: β = −0.192, P < 0.001; VitB12: β = −0.008, P < 0.001, respectively). It also showed that monotherapy with VPA (vs. Control: β = 2.406, P = 0.023) or OXC (vs. Control: β = 1.968, P = 0.041) and genotypes of MTHFR rs1801133 TT (vs. CC: β = 6.334, P < 0.001; vs. CT: β = 6.516, P < 0.001), TCN2 rs1801198 CC (vs. GC: β = 1.91, P = 0.039) and folate receptor 1 (FOLR1) rs2071010 AA (vs. GA: β = 4.464, P = 0.031) were independent risk factors for higher Hcy levels. According to the standard partial regression coefficient, the MTHFR rs1801133 TT genotype had the greatest effect on Hcy levels among these factors, and VPA had a stronger effect on Hcy levels than OXC (Table 3).
Table 3.
Effects of AEDs, SNPs and vitamins on Hcy levels in patients with epilepsy.
| Model | Unstandardized coefficients | Standardized coefficients | P-value | VIF | R2 | |
|---|---|---|---|---|---|---|
| β | Std. error | Beta | ||||
| (Constant) | 17.645 | 1.465 | <0.001 | 0.28 | ||
| FA (nmol/l) | −0.192 | 0.032 | −0.33 | <0.001 | 1.125 | |
| vit B12 (pmol/l) | −0.008 | 0.002 | −0.205 | <0.001 | 1.156 | |
| VPA | 2.406 | 1.048 | 0.151 | 0.023 | 1.587 | |
| OXC | 1.968 | 0.957 | 0.137 | 0.041 | 1.628 | |
| LTG | 0.435 | 1.011 | 0.028 | 0.668 | 1.519 | |
| LEV | 1.421 | 1.157 | 0.075 | 0.22 | 1.379 | |
| rs1801133 CT | −0.182 | 0.719 | −0.014 | 0.801 | 1.141 | |
| rs1801133 TT | 6.334 | 1.424 | 0.242 | <0.001 | 1.09 | |
| rs1801198 CC | 1.91 | 0.92 | 0.119 | 0.039 | 1.204 | |
| rs1801198 GG | 0.716 | 0.745 | 0.054 | 0.337 | 1.168 | |
| rs2071010 AA | 4.464 | 2.063 | 0.119 | 0.031 | 1.113 | |
| rs2071010 GG | 1.207 | 0.727 | 0.09 | 0.098 | 1.089 |
The overall significance for Hcy levels was F = 8.583; P < 0.001. P < 0.05 was considered a statistically significant difference.
FA, folate; vit B12, vitamin B12; VPA, valproate; OXC, oxcarbazepine; LTG, lamotrigine; LEV, levetiracetam; VIF, variable inflation factor.
In the subgroup analysis of patients taking OXC, we found that genotypes of MTHFR rs1801133 TT (vs. CC: β = 13.282, P = 0.001; vs. CT: β = 14.814, P < 0.001) and TCN2 rs1801198 CC (vs. GC: β = 5.432, P = 0.021; vs. GG: β = 5.905, P = 0.018) resulted in higher serum Hcy levels (as shown in Table 4). However, similar relationships were not observed for patients on VPA. We suspected that MTHFR rs1801133 TT and TCN2 rs1801198 CC genotypes may make patients susceptible to the effect of OXC on increasing Hcy levels.
Table 4.
Effects of SNPs and vitamins on Hcy levels in patients with epilepsy receiving OXC monotherapy.
| Model | Unstandardized coefficients | Standardized coefficients | P-value | VIF | R2 | |
|---|---|---|---|---|---|---|
| β | Std. error | Beta | ||||
| (Constant) | 21.058 | 2.895 | <0.001 | 0.378 | ||
| rs1801133 CT | −1.534 | 1.746 | −0.092 | 0.383 | 1.141 | |
| rs1801133 TT | 13.282 | 3.729 | 0.370 | 0.001 | 1.124 | |
| rs1801198 CC | 5.432 | 2.297 | 0.246 | 0.021 | 1.127 | |
| rs1801198 GG | −0.473 | 1.838 | −0.027 | 0.798 | 1.125 | |
| FA (nmol/l) | −0.334 | 0.106 | −0.327 | 0.002 | 1.119 |
The overall significance for Hcy levels was F = 7.891; P < 0.001. P < 0.05 was considered a statistically significant difference.
FA, folate; VIF, variable inflation factor.
Discussion
The first generation enzyme-inducing antiepileptic drugs, such as phenytoin and carbamazepine, may cause a deficiency of folate by influencing the activity of the hepatic enzymes and hence increase Hcy levels (1, 28). Compared with them, the second generation AEDs are less likely to stimulate enzymes of the liver and then are supposed less likely to disrupt Hcy metabolism, however, the conclusions are still waiting to be drawn. In this study, phenytoin and carbamazepine were excluded, because none of the patients took phenytoin monotherapy and only four patients took carbamazepine monotherapy in our clinic practice, while VPA, OXC, LTG and LEV, the most commonly used antiepileptic drugs in monotherapy, were included in this study.
In our study, after adjusting for related risk factors, such as FA levels, vitamin B12 levels and some OCM-related enzyme SNPs, LTG and LEV monotherapy were innocent of increasing Hcy levels, while OXC and VPA were associated with increased Hcy levels in patients with epilepsy. Several published studies found that in patients stabilized on LTG and LEV monotherapy, blood Hcy level were not significantly different from those observerd in controls (28, 34), similar to our findings. Conversely, a prospective longitudinal study showed that 6 months of LEV and OXC monotherapy significantly increased Hcy levels in patients with newly diagnosed epilepsy who were drug-free at baseline (35). Another study found that OXC therapy was associated with increased Hcy levels, even after controlling for sex, age, vitamin B12 levels, FA levels and the MTHFR rs1801133 TT genotype (28). Regarding the effect of VPA on Hcy levels, previous work by our research members, including a meta-analysis and a previously published study, also suggested that VPA was associated with high Hcy levels (27, 36). However, a small-sample study showed that VPA had no effect on Hcy levels in children with epilepsy compared with healthy children (26). Thus, although AEDs, including OXC, are generally recognized to increase Hcy levels by interfering with important cofactors (e.g., FA and vitamin B12) in the OCM pathway (37), the effect of VPA on Hcy levels and the underlying mechanism have still not been completely clarified. As shown in this study, treatment with VPA monotherapy was associated with higher vitB12 levels than treatment with LTG, LEV, OXC or controls. The existing literature also shows that VPA-related increases in Hcy levels may not be reduced by FA and vitamin B12 supplementation within a certain range (1). According to Anna et al., vitamin supplementation, including folate (0.4 mg a day), magnesium with 50 mg of vitamin B6 and vitamin B12 (100 μg a day), in 23 VPA-treated patients with chronic epilepsy for 1 year significantly increases s-FA levels (before vs. after supplementation: 8.4 ± 4.2 vs. 9.7 ± 4.5 ng/ml, P = 0.04), but p-tHcy levels are not decreased (before vs. after supplementation: 9.8 ± 3.4 vs. 9.3 ± 1.4 μmol/l, P > 0.05) (1). Taken together, we speculate that the increased Hcy levels caused by AEDs are not entirely dependent on deficiencies in FA and vitamin B12 but also dependent on additional mechanisms that remain to be elucidated.
SNPs in OCM-related enzymes may also be involved in disturbing OCM by changing enzyme activity. MTHFR is the critical enzyme that catalyzes the transformation of 5,10-CH2-THF to 5-mTHF. 5-mTHF, the major circulating form of folate, acts as a C donor for the vitamin B12-dependent remethylation of Hcy to methionine (38). The MTHFR rs1801133 TT genotype is significantly associated with decreased MTHFR specific activity (38); therefore, it might increase Hcy levels and serve as a risk factor for congenital malformations, such as NTDs and cleft lip with or without cleft palate (CL/P) (30). In our study, serum Hcy levels in patients with the MTHFR rs1801133 TT genotype were significantly higher than in patients with the MTHFR rs1801133 CT or CC genotype, even after adjusting for multiple related risk factors. The MTHFR rs1801133 TT genotype was also shown to be an independent risk factor for increased blood Hcy levels in patients with epilepsy taking OXC monotherapy.
TCN2, a cobalamin-transporting protein, mediates the transmembrane transport of cobalamin, which is a key cofactor in the reaction catalyzing the methylation of Hcy to methionine (39). The clinical importance of polymorphisms in TCN2 rs1801198 remains controversial. A systematic review and meta-analysis showed no significant association between TCN2 rs1801198 and FA levels or primary risks of congenital abnormalities; however, in individuals of European descent, Hcy levels were significantly higher in subjects with the TCN2 rs1801198 GG genotype than in subjects with the TCN2 rs1801198 CC genotype (31). A family-based, candidate gene association study of non-syndromic cleft palate only (CPO), which included 129 Italian and 65 Asian families, found no evidence of an association between TCN2 rs1801198 and CPO (40). However, another study reported that the TCN2 rs1801198 GG genotype was associated with an increased risk of fetal cleft lip with or without cleft palate in Californian women with low folate intake, although the sample size was too small to obtain meaningful conclusions (41). In addition, Martinelli et al. also identified a causative role for the TCN2 rs1801198 GG genotype in CL/P in Italy (42); however, in another recently published study, the same research group reported that the TCN2 rs1801198 GG genotype was associated with a decreased risk of cleft in Iraqi children (43). In this study, we observed that the TCN2 rs1801198 CC genotype was an independent risk factor for higher Hcy levels not only in all patients with epilepsy (CC vs. GC: β = 1.91, P = 0.039) but also specifically in patients on OXC (CC vs. GC: β = 5.432, P = 0.021; CC vs. GG: β = 5.905, P = 0.018). Consequently, the effect of genetic polymorphisms in TCN2 rs1801198 on blood Hcy levels requires further studied.
FOLR1 is a high-affinity folate receptor that transports folate, preferably the oxidized form of folate, via receptor-mediated endocytosis (44). A recent study in India including 206 probands with autism spectrum disorder (ASD) and 286 age-matched controls revealed a higher occurrence of the FOLR1 rs2071010 AA genotype in the probands with ASD, more specifically in the male subjects, compared with gender-matched controls (P = 0.02; CI 1.28–32.64), thereby indicating a positive association of the FOLR1 rs2071010 AA genotype with ASD (45). Our study showed that FOLR1 rs2071010 AA genotype was an independent risk factor for higher Hcy levels.
Although a single key genetic factor may disturb Hcy homeostasis, gene-gene or gene-environment interactions may also be involved in Hcy metabolism. Subsequently, one of the limitations of this paper is that we only included 23 SNPs in the OCM pathway in this study, which is not an extensive list. Second, other environmental factors that might affect the results, such as the intake of dietary folate, were not excluded in this study. Third, although we choose patients with epilepsy not taking AEDs for at least 6 months as controls, which might help to exclude the effects of being epileptic on Hcy metabolism in our subject, however, lack of healthy controls may make our study less rigorous. Finally, the small sample size in this study was also a limitation and larger sample studies are warranted to validate our findings.
Conclusion
We investigated the effects of 23 SNPs in 13 genes encoding OCM-related enzymes and AED monotherapy (e.g., VPA, OXC, LTG or LEV) on blood Hcy levels in patients with epilepsy. Based on our results, VPA, OXC, and genotypes of MTHFR rs1801133 TT, TCN2 rs1801198 CC and FOLR1 rs2071010 AA are all independent risk factors for elevated Hcy levels. The MTHFR rs1801133 TT and TCN2 rs1801198 CC may be susceptible genotypes to increase blood Hcy levels in patients with epilepsy, especially when combined with OXC monotherapy. Thus, genotyping those three SNPs in patients with epilepsy, especially those who are taking OXC, might be of a certain significance in guiding clinical medications.
Data Availability Statement
The data presented in the study are deposited in the dbSNP repository: https://www.ncbi.nlm.nih.gov/SNP/snp_viewTable.cgi?handle=FAHOSYSU.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
SZ: conceptualization, methodology, investigation, and writing– original draft. GN: methodology, formal analysis, and investigation. LS: resources, data curation, and writing– review and editing. YZ: validation and software. XZ: software and formal analysis. QD: resources and writing– review and editing. AC: software and data curation. WL: data curation. YL: visualization. MH: resources. LZ: writing– review and editing, supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the financial support received from the National Natural Science Foundation of China and Sanming Project of Medicine in Shenzhen. The authors acknowledge Shan Zhou and Shaoxing Guan for the technical assistance. The authors thank all participants for their participation.
Glossary
Abbreviations
- SNPs
some single nucleotide polymorphisms
- OCM
one-carbon metabolism
- AED
antiepileptic drug
- Hcy
homocysteine
- VPA
valproate
- OXC
oxcarbazepine
- LTG
lamotrigine
- LEV
levetiracetam
- vit B12
vitamin B12
- FA
folate
- MTHFR
methylenetetrahydrofolate reductase
- TCN2
transcobalamin 2
- FOLR1
folate receptor 1
- NTDs
neural tube defects
- CBS
cystathionine synthase
- 5-mTHF
5-methyltetrahydrofolate
- 5, 10-CH2-THF, 5
10-methylenetetrahydrofolate
- BHMT
betaine homocysteine methyltransferase
- MAT
methionine adenosyltransferase
- SAM
s-adenosylmethionine
- SAH
s-adenosylhomocysteine
- MTHFD1
methylenetetrahydrofolate dehydrogenase 1
- ANOVA
one-way analysis of variance
- CL/P
cleft lip with or without cleft palate
- CPO
nonsyndromic cleft palate only
- ASD
autism spectrum disorder.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (81571266, 81771405, 82071447) and Sanming Project of Medicine in Shenzhen (No. SZSM201911003).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.683275/full#supplementary-material
References
- 1.Bochynska A, Lipczynska-Lojkowska W, Gugala-Iwaniuk M, Lechowicz W, Restel M, Graban A, et al. The effect of vitamin B supplementation on homocysteine metabolism and clinical state of patients with chronic epilepsy treated with carbamazepine and valproic acid. Seizure. (2012) 21:276–81. 10.1016/j.seizure.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 2.Verrotti A, Pascarella R, Trotta D, Giuva T, Morgese G, Chiarelli F. Hyperhomocysteinemia in children treated with sodium valproate and carbamazepine. Epilepsy Res. (2000) 41:253–7. 10.1016/S0920-1211(00)00150-9 [DOI] [PubMed] [Google Scholar]
- 3.Caccamo D, Condello S, Gorgone G, Crisafulli G, Belcastro V, Gennaro S, et al. Screening for C677T and A1298C MTHFR polymorphisms in patients with epilepsy and risk of hyperhomocysteinemia. Neuromol Med. (2004) 6:117–26. 10.1385/NMM:6:2-3:117 [DOI] [PubMed] [Google Scholar]
- 4.Belcastro V, Gaetano G, Italiano D, Oteri G, Caccamo D, Pisani LR, et al. Antiepileptic drugs and MTHFR polymorphisms influence hyper-homocysteinemia recurrence in epileptic patients. Epilepsia. (2007) 48:1990–4. 10.1111/j.1528-1167.2007.01164.x [DOI] [PubMed] [Google Scholar]
- 5.Cavalca V, Cighetti G, Bamonti F, Loaldi A, Bortone L, Novembrino C, et al. Oxidative stress and homocysteine in coronary artery disease. Clin Chem. (2001) 47:887–92. 10.1093/clinchem/47.5.887 [DOI] [PubMed] [Google Scholar]
- 6.Soinio M, Marniemi J, Laakso M, Lehto S, Ronnemaa T. Elevated plasma homocysteine level is an independent predictor of coronary heart disease events in patients with type 2 diabetes mellitus. Ann Intern Med. (2004) 140:94–100. 10.7326/0003-4819-140-2-200401200-00009 [DOI] [PubMed] [Google Scholar]
- 7.van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der Klift M, de Jonge R, Lindemans J, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. (2004) 350:2033–41. 10.1056/NEJMoa032546 [DOI] [PubMed] [Google Scholar]
- 8.Mclean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, et al. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med. (2004) 350:2042–9. 10.1056/NEJMoa032739 [DOI] [PubMed] [Google Scholar]
- 9.Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res. (2005) 20:921–9. 10.1359/JBMR.050202 [DOI] [PubMed] [Google Scholar]
- 10.Tanne D, Haim M, Goldbourt U, Boyko V, Doolman R, Adler Y, et al. Prospective study of serum homocysteine and risk of ischemic stroke among patients with preexisting coronary heart disease. Stroke. (2003) 34:632–6. 10.1161/01.STR.0000060203.58958.35 [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Sun L, Zhang H, Liao Y, Wang D, Zhao B, et al. Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: a Multicenter Case-Control Study in China. Stroke. (2003) 34:2085–90. 10.1161/01.STR.0000086753.00555.0D [DOI] [PubMed] [Google Scholar]
- 12.Iso H, Moriyama Y, Sato S, Kitamura A, Tanigawa T, Yamagishi K, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. (2004) 109:2766–72. 10.1161/01.CIR.0000131942.77635.2D [DOI] [PubMed] [Google Scholar]
- 13.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. (2002) 346:476–83. 10.1056/NEJMoa011613 [DOI] [PubMed] [Google Scholar]
- 14.Morris MS. Homocysteine and Alzheimer's disease. Lancet Neurol. (2003) 2:425–8. 10.1016/S1474-4422(03)00438-1 [DOI] [PubMed] [Google Scholar]
- 15.Mills JL, Mcpartlin JM, Kirke PN, Lee YJ, Conley MR, Weir DG, et al. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet. (1995) 345:149–51. 10.1016/S0140-6736(95)90165-5 [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Diaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. (2012) 78:1692–9. 10.1212/WNL.0b013e3182574f39 [DOI] [PubMed] [Google Scholar]
- 17.Zhao W, Mosley BS, Cleves MA, Melnyk S, James SJ, Hobbs CA. Neural tube defects and maternal biomarkers of folate, homocysteine, and glutathione metabolism. Birth Defects Res A Clin Mol Teratol. (2006) 76:230–6. 10.1002/bdra.20240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldelli E, Leo G, Andreoli N, Fuxe K, Biagini G, Agnati LF. Homocysteine potentiates seizures and cell loss induced by pilocarpine treatment. Neuromol Med. (2010) 12:248–59. 10.1007/s12017-009-8110-1 [DOI] [PubMed] [Google Scholar]
- 19.Kubova H, Folbergrova J, Mares P. Seizures induced by homocysteine in rats during ontogenesis. Epilepsia. (1995) 36:750–6. 10.1111/j.1528-1157.1995.tb01611.x [DOI] [PubMed] [Google Scholar]
- 20.Rezaei S, Shab-Bidar S, Abdulahi AA, Djafarian K. Oxcarbazepine administration and the serum levels of homocysteine, vitamin B12 and folate in epileptic patients: a systematic review and meta-analysis. Seizure. (2017) 45:87–94. 10.1016/j.seizure.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 21.Schalinske KL, Smazal AL. Homocysteine imbalance: a pathological metabolic marker. Adv Nutr. (2012) 3:755–62. 10.3945/an.112.002758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Zhu H, Fu G, Wang M, Zhang Z, Lu Q, et al. Polymorphisms of methylenetetrahydrofolate reductase and methionine synthase genes and bladder cancer risk: a case-control study with meta-analysis. Clin Exp Med. (2009) 9:9–19. 10.1007/s10238-008-0013-1 [DOI] [PubMed] [Google Scholar]
- 23.Amorim MR, Moura CM, Gomes AD, Barboza HN, Lopes RB, Ribeiro MG, et al. Betaine-homocysteine methyltransferase 742G>A polymorphism and risk of down syndrome offspring in a Brazilian population. Mol Biol Rep. (2013) 40:4685–9. 10.1007/s11033-013-2563-x [DOI] [PubMed] [Google Scholar]
- 24.Bharatkumar VP, Rudreshkumar KJ, Nagaraja D, Christopher R. Plasma S-adenosylhomocysteine: a potential risk marker for cerebral venous thrombosis. Clin Chim Acta. (2016) 458:44–8. 10.1016/j.cca.2016.04.024 [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T, Scheet P, Giusti B, Bandinelli S, Piras MG, Usala G, et al. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet. (2009) 84:477–82. 10.1016/j.ajhg.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emeksiz HC, Serdaroglu A, Biberoglu G, Gulbahar O, Arhan E, Cansu A, et al. Assessment of atherosclerosis risk due to the homocysteine-asymmetric dimethylarginine-nitric oxide cascade in children taking antiepileptic drugs. Seizure. (2013) 22:124–7. 10.1016/j.seizure.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 27.Ni G, Qin J, Chen Z, Li H, Zhou J, Huang M, et al. Associations between genetic variation in one-carbon metabolism and leukocyte DNA methylation in valproate-treated patients with epilepsy. Clin Nutr. (2018) 37:308–12. 10.1016/j.clnu.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 28.Belcastro V, Striano P, Gorgone G, Costa C, Ciampa C, Caccamo D, et al. Hyperhomocysteinemia in epileptic patients on new antiepileptic drugs. Epilepsia. (2010) 51:274–9. 10.1111/j.1528-1167.2009.02303.x [DOI] [PubMed] [Google Scholar]
- 29.Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust. (2018) 208:226–33. 10.5694/mja17.00951 [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Qi J, Yu X, Zhu J, Zhang L, Ning Q, et al. Investigations of single nucleotide polymorphisms in folate pathway genes in Chinese families with neural tube defects. J Neurol Sci. (2014) 337:61–6. 10.1016/j.jns.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 31.Oussalah A, Levy J, Filhine-Tresarrieu P, Namour F, Gueant JL. Association of TCN2 rs1801198 c.776G>C polymorphism with markers of one-carbon metabolism and related diseases: a systematic review and meta-analysis of genetic association studies. Am J Clin Nutr. (2017) 106:1142–56. 10.3945/ajcn.117.156349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sniezawska A, Dorszewska J, Rozycka A, Przedpelska-Ober E, Lianeri M, Jagodzinski PP, et al. MTHFR, MTR, and MTHFD1 gene polymorphisms compared to homocysteine and asymmetric dimethylarginine concentrations and their metabolites in epileptic patients treated with antiepileptic drugs. Seizure. (2011) 20:533–40. 10.1016/j.seizure.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 33.Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. (2009) Chapter 2:Unit 2.12. 10.1002/0471142905.hg0212s60 [DOI] [PubMed] [Google Scholar]
- 34.Ni G, Qin J, Li H, Chen Z, Zhou Y, Fang Z, et al. Effects of antiepileptic drug monotherapy on one-carbon metabolism and DNA methylation in patients with epilepsy. PLoS ONE. (2015) 10:e0125656. 10.1371/journal.pone.0125656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim DW, Lee SY, Shon YM, Kim JH. Effects of new antiepileptic drugs on circulatory markers for vascular risk in patients with newly diagnosed epilepsy. Epilepsia. (2013) 54:e146–9. 10.1111/epi.12338 [DOI] [PubMed] [Google Scholar]
- 36.Ni G, Qin J, Fang Z, Chen Y, Chen Z, Zhou J, et al. Increased homocysteine levels in valproate-treated patients with epilepsy: a meta-analysis. BMJ Open. (2014) 4:e004936. 10.1136/bmjopen-2014-004936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulido FL, Pulido FM, Quesada JP, Muruzabal PJ, Mendioroz IM. Comparative case-control study of homocysteine, vitamin B12, and folic acid levels in patients with epilepsy. Neurologia. (2017) 32:440–5. 10.1016/j.nrleng.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 38.Chango A, Boisson F, Barbe F, Quilliot D, Droesch S, Pfister M, et al. The effect of 677C–>T and 1298A–>C mutations on plasma homocysteine and 5,10-methylenetetrahydrofolate reductase activity in healthy subjects. Br J Nutr. (2000) 83:593–6. 10.1017/S0007114500000751 [DOI] [PubMed] [Google Scholar]
- 39.Balduino VD, de Godoy MF, Goloni-Bertollo EM, Pavarino EC. Genetic polymorphisms involved in folate metabolism and maternal risk for down syndrome: a meta-analysis. Dis Mark. (2014) 2014:517504. 10.1155/2014/517504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carinci F, Palmieri A, Scapoli L, Cura F, Borelli F, Morselli PG, et al. Non-syndromic cleft palate: association analysis on three gene polymorphisms of the folate pathway in Asian and Italian populations. Int J Immunopathol Pharmacol. (2019) 33:2058738419858572. 10.1177/2058738419858572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marini NJ, Yang W, Asrani K, Witte JS, Rine J, Lammer EJ, et al. Sequence variation in folate pathway genes and risks of human cleft lip with or without cleft palate. Am J Med Genet A. (2016) 170:2777–87. 10.1002/ajmg.a.37874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinelli M, Scapoli L, Palmieri A, Pezzetti F, Baciliero U, Padula E, et al. Study of four genes belonging to the folate pathway: transcobalamin 2 is involved in the onset of non-syndromic cleft lip with or without cleft palate. Hum Mutat. (2006) 27:294. 10.1002/humu.9411 [DOI] [PubMed] [Google Scholar]
- 43.Carinci F, Palmieri A, Scapoli L, Cura F, Abenavoli F, Gianni AB, et al. Association between oral cleft and transcobalamin 2 polymorphism in a sample study from Nassiriya, Iraq. Int J Immunopathol Pharmacol. (2019) 33:2058738419855571. 10.1177/2058738419855571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurosawa Y, Furugen A, Nishimura A, Narumi K, Kobayashi M, Iseki K. Evaluation of the effects of antiepileptic drugs on folic acid uptake by human placental choriocarcinoma cells. Toxicol In Vitro. (2018) 48:104–10. 10.1016/j.tiv.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 45.Saha S, Saha T, Sinha S, Rajamma U, Mukhopadhyay K. Autistic traits and components of the folate metabolic system: an explorative analysis in the eastern Indian ASD subjects (dagger). Nutr Neurosci. (2020) 23:860–7. 10.1080/1028415X.2019.1570442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in the study are deposited in the dbSNP repository: https://www.ncbi.nlm.nih.gov/SNP/snp_viewTable.cgi?handle=FAHOSYSU.