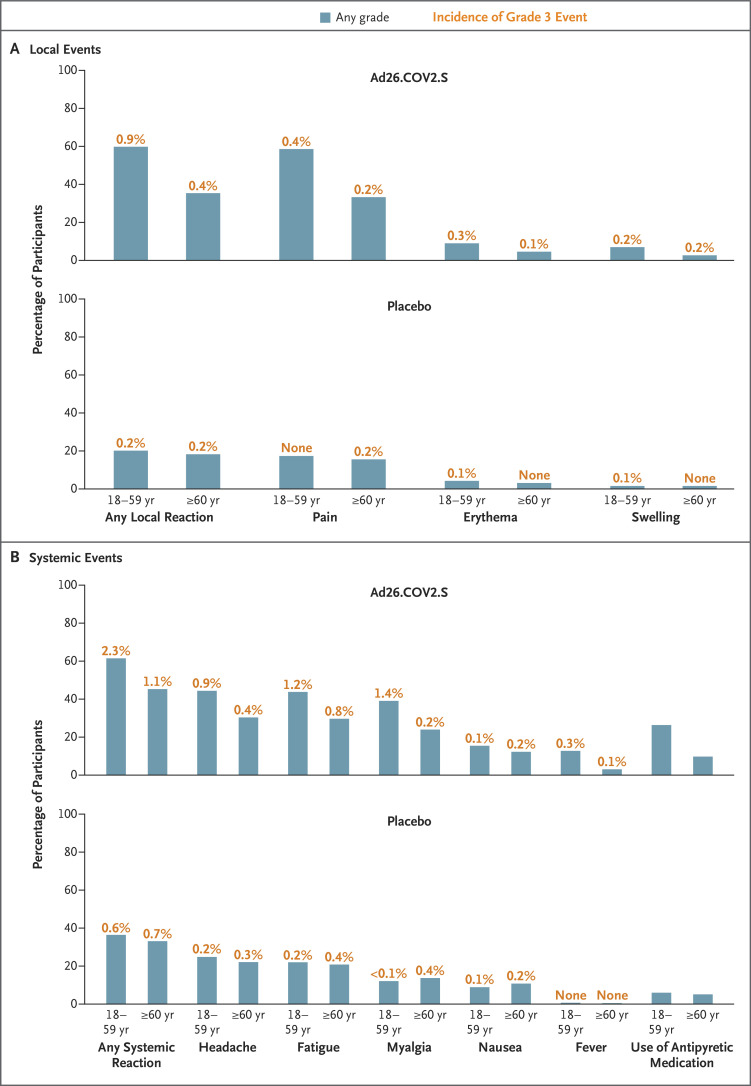
Figure 1. Solicited Local and Systemic Adverse Events Reported within 7 days after the Administration of Vaccine or Placebo (Safety Subpopulation).
Most solicited local and systemic adverse events occurred within 1 to 2 days after the administration of vaccine or placebo and had a median duration of 1 to 2 days. No grade 4 local or systemic adverse events were reported. There were no local or systemic reactogenicity differences between participants who were seronegative at baseline and those who were seropositive (data not shown). Pain was categorized as grade 1 (mild; does not interfere with activity), grade 2 (moderate; requires modification of activity or involves discomfort with movement), grade 3 (severe; inability to perform usual activities), or grade 4 (potentially life-threatening; hospitalization or inability to perform basic self-care). Erythema and swelling were categorized as grade 1 (mild; 25 to 50 mm), grade 2 (moderate; 51 to 100 mm), grade 3 (severe; >100 mm), or grade 4 (potentially life-threatening; necrosis or leading to hospitalization). Systemic events were categorized as grade 1 (mild; minimal symptoms), grade 2 (moderate; notable symptoms not resulting in loss of work or school time), grade 3 (severe; incapacitating symptoms resulting in loss of work or school time), or grade 4 (life-threatening; hospitalization or inability to perform basic self-care). Fever was defined as grade 1 (mild; ≥38.0 to 38.4°C), grade 2 (moderate; ≥38.5 to 38.9°C), grade 3 (severe; ≥39.0 to 40.0°C), or grade 4 (potentially life-threatening; >40°C).