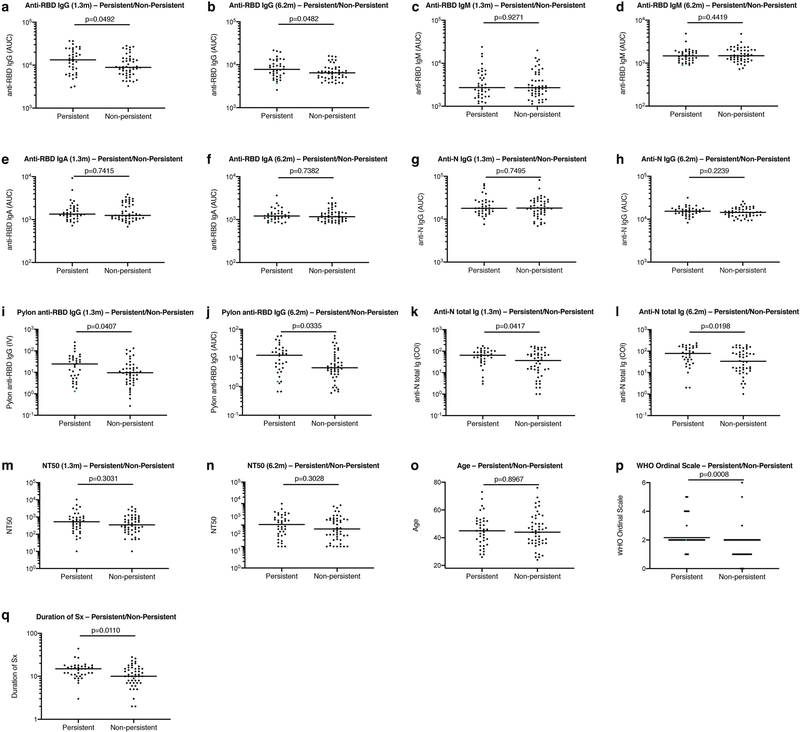
Extended Data Fig. 1 |. Clinical correlates of plasma antibody titres.
a, Normalized AUC anti-RBD IgG titres at 1.3 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. b, Normalized AUC anti-RBD IgG titres at 6.2 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. c, Normalized AUC anti-RBD IgM titres at 1.3 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. d, Normalized AUC anti-RBD IgM titres at 6.2 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. e, Normalized AUC anti-RBD IgA titres at 1.3 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. f, Normalized AUC anti-RBD IgA titres at 6.2 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. g, Normalized AUC anti-N IgG titres at 1.3 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. h, Normalized AUC anti-N IgG titres at 6.2 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. i, Index values (IV) of anti-RBD IgG titres at 1.3 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. j, Index values of anti-RBD IgG titres at 6.2 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. k, Cut-off index (COI) values of anti-N total Ig titres at 1.3 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. l, COI values of anti-N total Ig titres at 6.2 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. m, NT50 values at 1.3 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. n, NT50 values at 6.2 months for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. o, Age in years for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. p, Severity of acute infection as assessed by the WHO ‘Ordinal Clinical Progression/Improvement Scale’ for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. q, Duration of symptoms during acute infection for participants with (n = 38) or without (n = 49) persistent post-acute symptoms. Horizontal bars indicate median values. Statistical significance was determined using two-tailed Mann–Whitney U-tests.