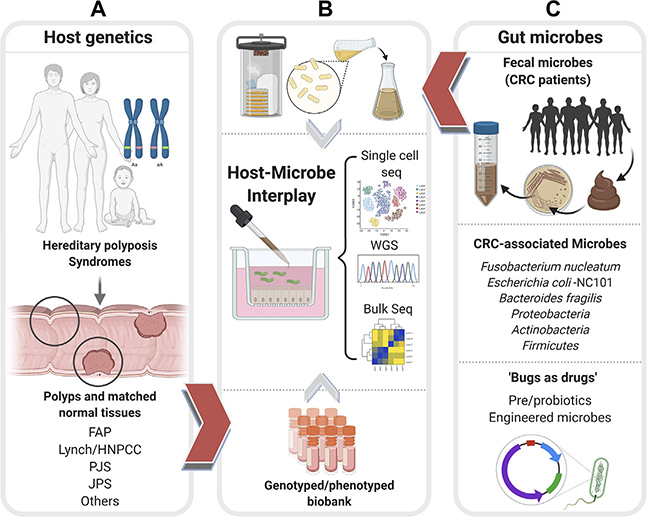
Fig. 3.
Patient-derived organoids can enable an objective assessment of the relative contributions of host genetics and microbes toward CRC initiation, reveal the potential use of microbes as therapeutics. Schematic displays how patient-derived organoids (PDOs) from uninvolved normal mucosal biopsies and involved adenomatous regions of colons representing diverse CRC syndromes (A), e.g., Familial adenomatous polyposis (FAP), Lynch or Hereditary nonpolyposis colorectal cancer (HNPCC), Peutz-Jeghers syndrome (PJS), Juvenile polyposis syndrome (JPS) of known genotypes can be used to represent host contributions in organoid-microbe coculture models in panel (B). PDOs can also help interrogate and assess the impact of both harmful and beneficial gut microbes (C). Fecal microbes collected from high-risk patients with CRCs/excessive polyposis (C, top) can be cultured using aerobic or anaerobic conditions (B, top) prior to use in coculture studies with normal PDOs. Bacterial strains associated with CRCs (C, middle) can similarly be used in cocultures with PDOs with known genotypes. Pre- and probiotics and engineered microbes (C, bottom) can be similarly tested for their beneficial effects on PDOs. In each coculture model, the PDOs can be objectively assessed in an unbiased way using “omics”- and computational approaches (B, middle) to measure the impact of host genetics, microbes, individually and when combined.