Figure 4:
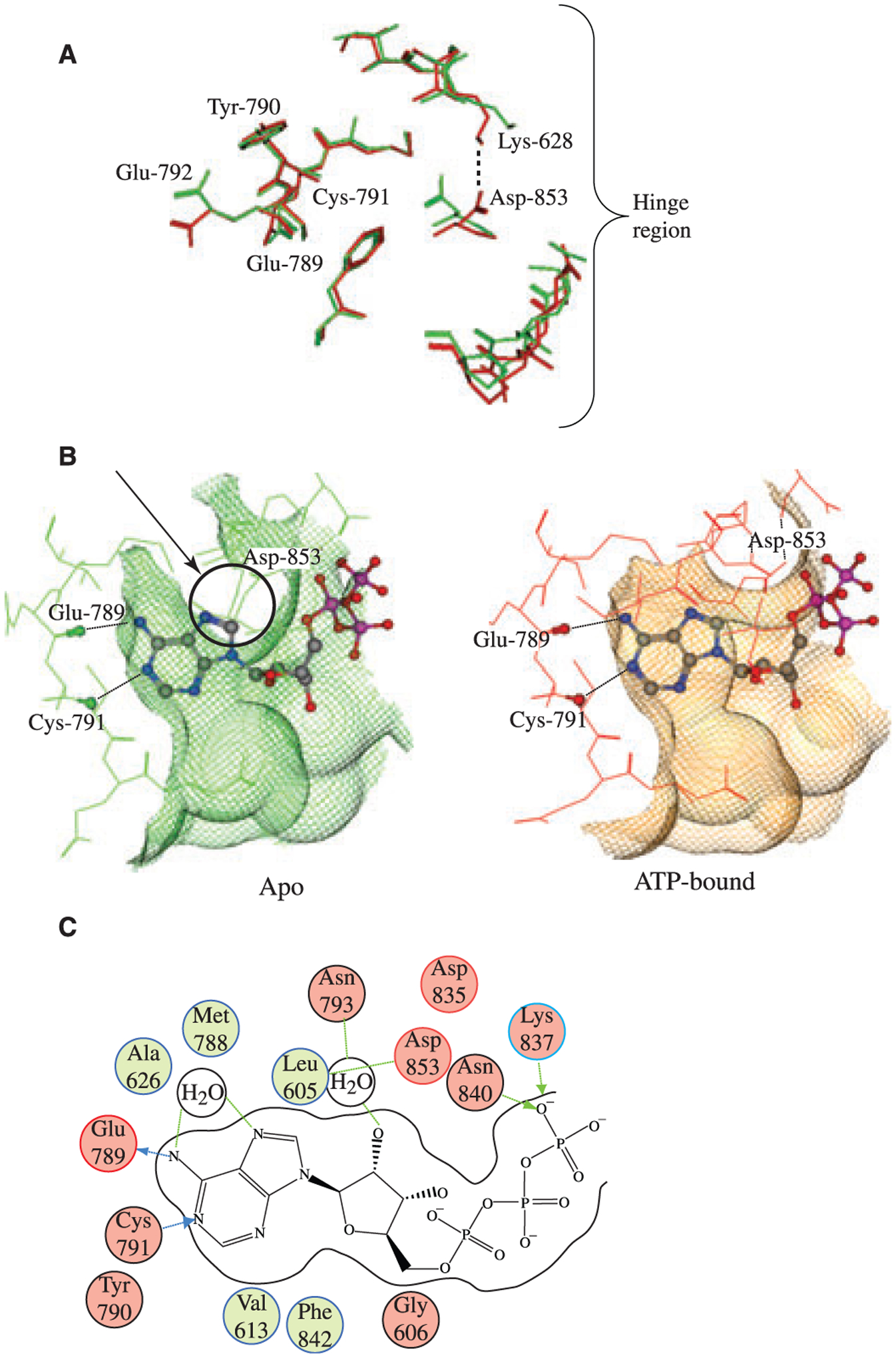
Analysis of the GCN2 ATP binding pocket. (A) Comparison of apo (green) and ATP bound (red) GCN2 structures. The most striking feature of pocket motion upon binding is the rotation of ASP-853 towards LYS-628, which opens up the binding pocket in the hinge region and allows ATP to bind. (B) Top view of apo (red) and ATP bound (green) GCN2 superimposed with bound ATP. In apo GCN2 the ASP-853 residue is in a conformation that would sterically clash with ATP. In order to bind ATP, the ASP-853 residue (indicated by an arrow and circled) must rotate out of position in order to open up the pocket around the hinge region, as demonstrated by the ATP bound structure (green). The conformation of the ASP-853 residue in the bound GCN2 structure may be partially stabilized by ionic/H-bond interaction with LYS-628; upon binding, LYS-628 rotates away from its conformation in the apo GCN2 structure and adopts a conformation more suitable for interaction with ASP-853. (C) 2D diagram of ATP interactions with GCN2. The canonical ligand H-bonds to the hinge region are made with the backbone atoms of GLU-789 and CYS-791. The diagram also highlights the water-mediated H-bonds between the purine ring on ATP and the ASP-853 and MET-788 residues backbone. This water molecule is conserved in both the apo and the bound GCN2 structures.
