Figure 2.
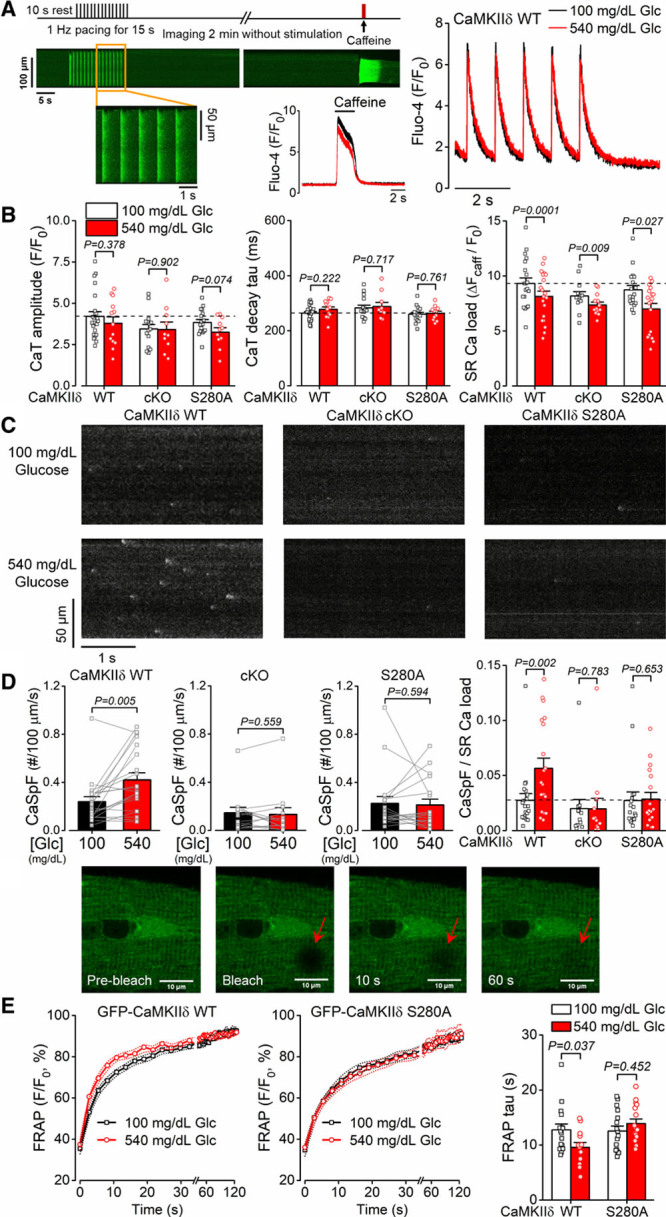
Glucose-induced diastolic sarcoplasmic reticulum (SR) Ca2+ leak is CaMKIIδ (Ca2+/calmodulin-dependent protein kinase II)-S280 O-GlcNAcylation dependent. A, Experimental protocol and representative intracellular Ca2+ signals (quantified as changes in Fluo-4 fluorescence). B, High-glucose treatment (540 mg/dL, 6 min) did not change the amplitude and decay of intracellular Ca2+ transient (CaT) but reduced SR Ca2+ load similarly in CaMKIIδ wild type (WT), cardiac-specific knockout (cKO), and O-GlcNAc-resistant CaMKIIδ-S280A knock-in (n[CaT]=total number of cells/animals, WT/normal glucose=24/8, WT/high-glucose=13/8, cKO/normal glucose=15/7, cKO/high-glucose=10/7, S280A/normal glucose=17/6, S280A/high-glucose=11/6; n[SR load]= total number of cells/animals, WT=20/11, cKO=13/9, S280A=18/9). Nested t test. C, Representative diastolic Ca2+ sparks were increased by high-glucose treatment in WT which was prevented in cKO and S280A. D, Increased Ca2+ spark frequency normalized to SR load indicates sensitized, leaky ryanodine receptors (n=total number of cells/animals, WT=20/11, cKO=13/9, S280A=18/9). Nested t test. E, Enhanced fluorescence recovery after photobleaching (FRAP) by high-glucose treatment in cardiomyocytes expressing GFP (green fluorescent protein)-tagged WT-CaMKIIδ (n=16 cells from 6 animals in normal glucose and n=13 cells from 6 animals in high-glucose) but not in GFP-CaMKIIδ-S280A (n=16 cells from 6 animals in both normal and high-glucose) indicates increased activation-dependent mobility of CaMKIIδ. Nested t test.
