Figure 3.
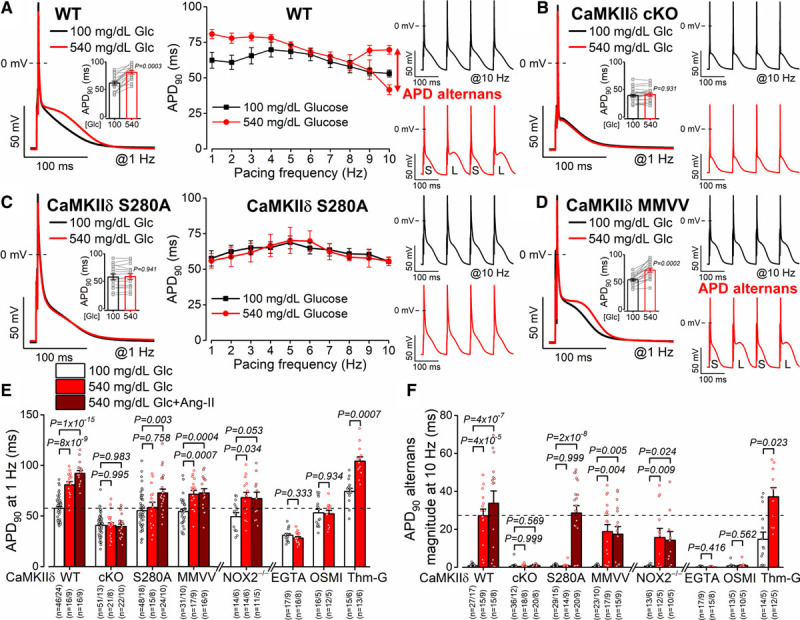
Glucose-induced arrhythmogenic action potentials are CaMKIIδ (Ca2+/calmodulin-dependent protein kinase II)-S280 O-GlcNAcylation dependent. A, Action potential duration (APD) prolongation and alternans (S, short; L, long) were increased by acute high-glucose treatment in wild-type (WT) myocytes (n=16 cells from 9 animals). B, Arrhythmogenic APD responses were prevented by CaMKIIδ-cardiac-specific knockout (cKO; n=21 cells from 8 animals). C and D, CaMKIIδ-S280A knock-in (n=15 ells from 8 animals) but not mutated Met281Val and Met282Val (MMVV; n=17 cells from 9 animals) was resistant to glucose-induced acute APD changes. Nested t test. E and F, Glucose-induced APD changes were prevented by CaMKIIδ-cKO, S280A, intracellular Ca2+ buffering (EGTA) or the O-GlcNAc transferase inhibitor OSMI-1 (αR-[[(1,2-dihydro-2-oxo-6-quinolinyl)sulfonyl]amino]-N-(2-furanylmethyl)-2-methoxy-N-(2-thienylmethyl)-benzeneacetamide) but enhanced by the O-GlcNAcase inhibitor Thiamet-G (Thm-G) in WT. Ang II (angiotensin II) further enhanced arrhythmogenic APD changes, which was prevented by CaMKIIδ-cKO, MMVV, or in NOX2−/− (NADPH oxidase 2; n=total number of cells/animals is reported in the figure). Nested 1-way ANOVA, followed by Dunnett multiple comparisons test was used to compare 3 groups. Nested t test was used to compare 2 groups.
