Figure 1.
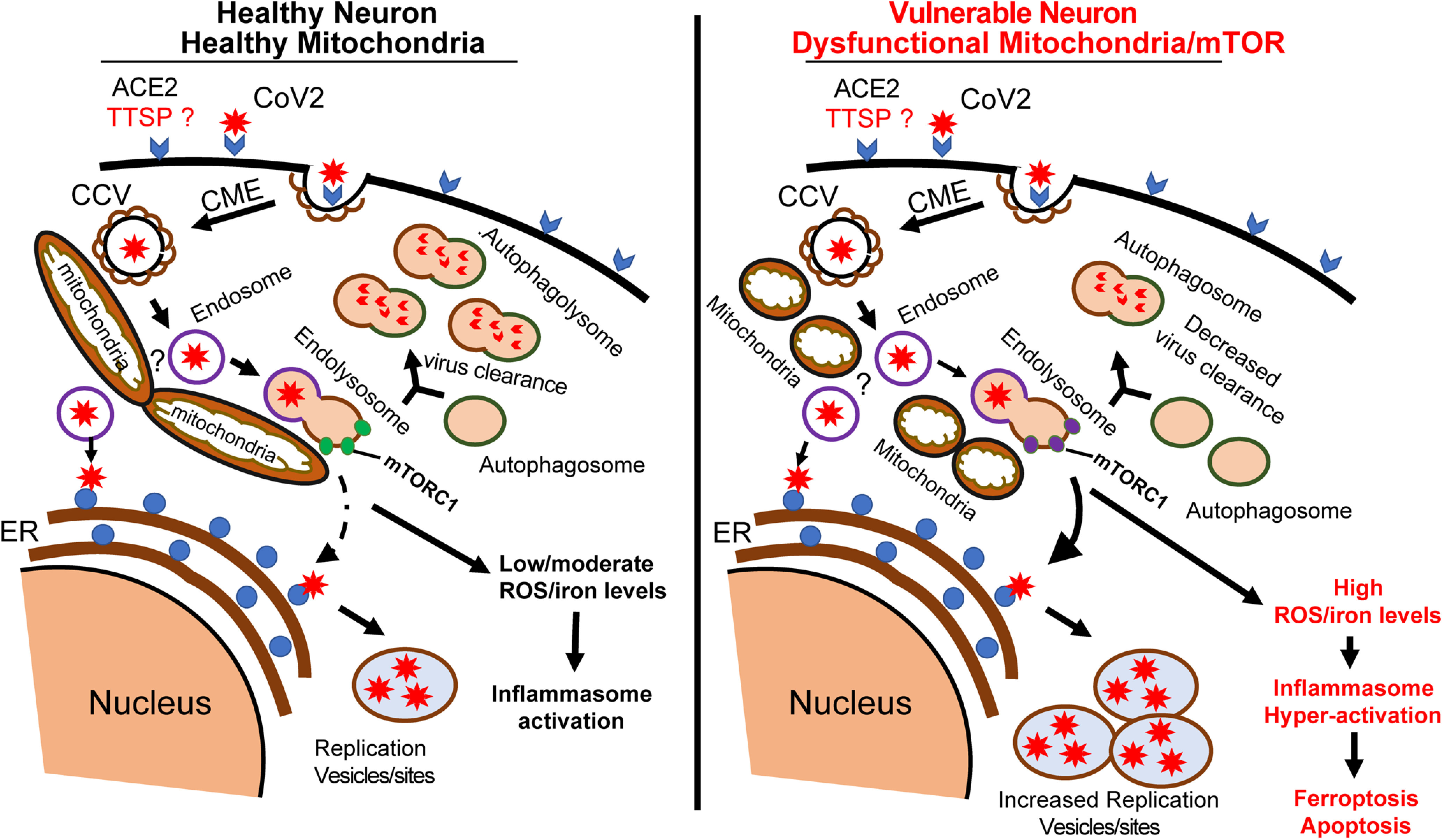
Schematic diagram of proposed SARS-CoV-2 entry into neurons and the role of mitochondria. In a healthy neuron, the initial binding of SARS-CoV-2 to receptors and unknown neuronal TTSP complex initiate CME. CCV containing the virus fuses with endosomes to expose the virus to the endoplasmic reticulum (ER) to make new viral proteins for viral replication. We hypothesize that at the mitochondria, the endosomes fuse with mTORC1-bound lysosomes to form a hybrid endolysosome, which can also interact with ER or fuse with autophagosomes in the autophagy pathway. The lysosome–autophagy pathway is active in healthy neurons, allowing degradation of CoV vesicles and virus clearance. In high-risk COVID patients, neurons have dysfunctional mitochondria and the lysosome–autophagy signaling is impaired, leading to increased virus replication and ROS/iron levels. The high levels of ROS/iron production initiate the induction of pathways such as apoptosis or ferroptosis.
