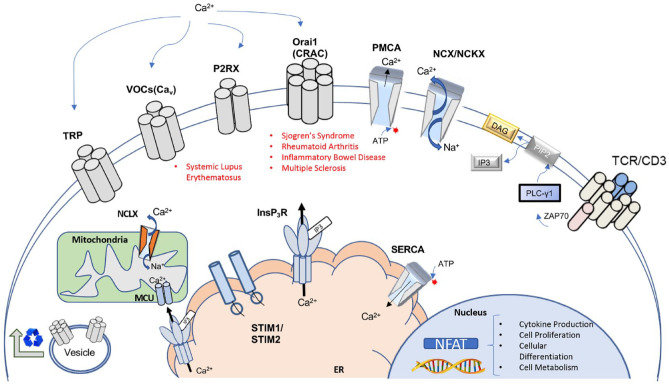
Figure 2.
Overview of major calcium channels and regulators of calcium signaling in T lymphocytes. Key components of Ca2+ homeostasis and signaling in T lymphocytes. Examples of autoimmune diseases currently associated with specific channelopathies are listed beneath respective constituents. Various channels are involved in homeostasis of Ca2+ as well as signaling events in receptor-mediated Ca2+ entry. Ca2+ influx is controlled via several main channel types: transient receptor potential (TRP) channels, voltage-gated Ca2+ channels (VOCs), purinergic ionotropic receptors (P2RXs), and calcium release-activated calcium channels (CRACs). Recycling and trafficking of channels to the plasma membrane occurs through vesicle-mediated transport from the Golgi apparatus. Transporters and ion pumps facilitate maintenance of cellular Ca2+ levels. Plasma membrane Ca2+ ATPases (PMCAs) and Na+/Ca2+ exchangers (NCKX) control efflux of cytoplasmic calcium. In the endoplasmic reticulum (ER), the sarcoplasmic/ER Ca2+ ATPase (SERCA) mediates calcium influx. T-cell receptor (TCR) stimulation generates IP3, leading to the release of ER calcium pools via InsP3 receptor (InsP3R) channels. Depletion of ER calcium stores is detected through EF-handed stromal interaction molecules 1 and 2 (STIM1, STIM2), which leads to sustained cytoplasmic Ca2+ influx via activation of Orai (CRAC channels) during T-cell activation. Contact sites between InsP3Rs and the mitochondrial Ca2+ uniporter (MCU) enable coupling of TCR stimulation to metabolic activities in the mitochondria. Mitochondrial Na+/Ca2+/Li+ exchangers (NCLXs) regulate mitochondrial Ca2+ levels. Transduction of TCR stimulation via Ca2+ signaling culminates in the activation of nuclear factor of activated T cells (NFATs). Activation of NFAT controls cellular responses associated with T-cell activation, including cytokine production, cell differentiation, and proliferation. Modified and expanded from Trebak and Kinet (2019) and Park et al. (2020).