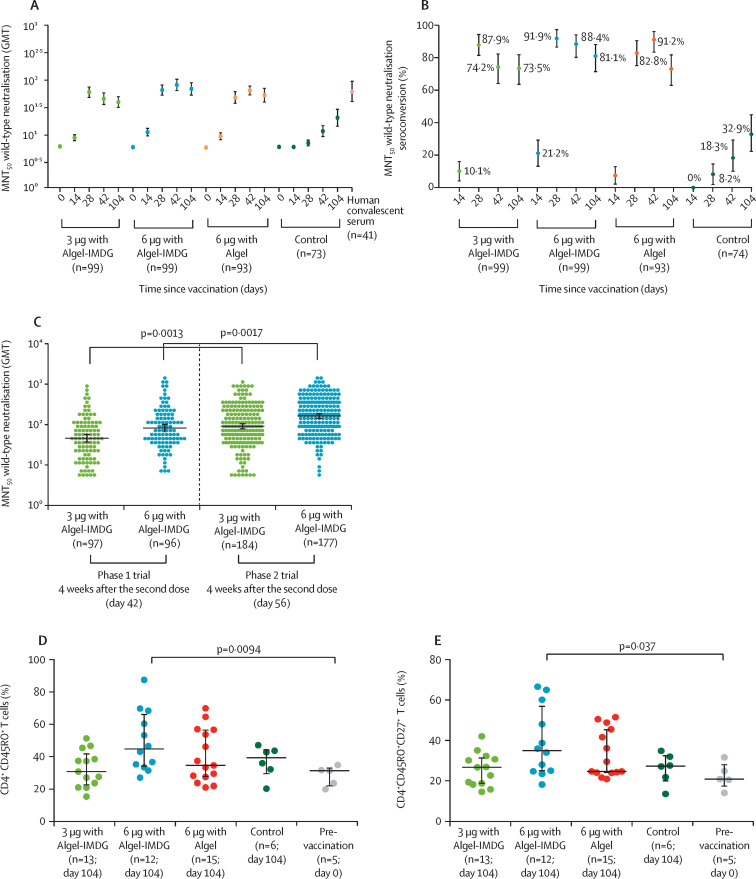
Figure 4.
SARS-CoV-2 wild-type MNT50 GMTs (A) and seroconversion rates (B) in phase 1 participants, SARS-CoV-2 wild-type MNT50 GMTs in phase 1 and phase 2 participants at 4 weeks after the second vaccination (C), and the proportion of CD4+ CD45RO+ (D) and CD4+ CD45RO+ CD27+ (E) T cells at day 104 in phase 1 participants
In the phase 1 trial, the dosing schedule was day 0 for the first dose of the vaccine and day 14 for the second dose. In the phase 2 trial, the dosing schedule was day 0 for the first dose of the vaccine and day 28 for the second dose. In A, results at baseline (day 0), 2 weeks after the second vaccination (day 28), 4 weeks after the second vaccination (day 42), and 3 months after the second vaccination (day 104) for the 3 μg and 6 μg with Algel-IMDG groups, the 6 μg with Algel group, and the Algel-only control group in the phase 1 trial are shown. The human convalescent serum panel included specimens from participants with PCR-confirmed symptomatic and asymptomatic COVID-19 obtained at least at least 30 days after diagnosis (41 samples). In B, seroconversion rates were defined by the proportion of post-vaccination titres that were at least four-fold higher than baseline. In D and E, the frequencies of antigen-specific T-cell memory responses at 3 months after the second dose (day 104) in all groups from the phase 1 trial are shown; dots are individual datapoints, and horizontal bars are medians with error bars for IQRs. GMT=geometric mean titre. MNT50=microneutralisation assay.