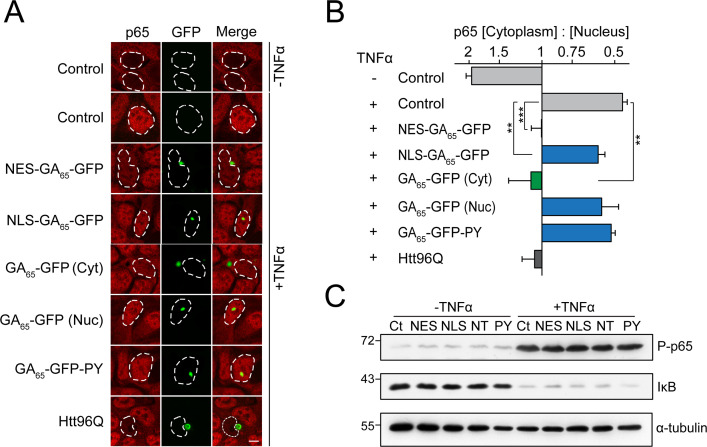
Figure 4. Cytoplasmic poly-GA aggregates inhibit nuclear import of p65.
(A) Cytoplasmic poly-GA aggregates inhibit p65 nuclear translocation. HEK293 cells were transfected with empty vector (Control), NES-GA65-GFP, NLS-GA65-GFP, GA65-GFP, GA65-GFP-PY, or Htt96Q-GFP (Htt96Q) (green) and analyzed for NF-κB p65 localization (red) with and without TNFα treatment (30 min). White dashed lines delineate nuclei based on DAPI staining. Scale bar represents 10 µm. (B) Quantification of NF-κB p65 distribution from data in (A). The x-axis shows the enrichment of p65 in the cytoplasm relative to the nucleus. Data are means + SD (n = 3), >100 cells were analyzed per condition. **p≤0.01, ***p≤0.001 from two-sided t-test. (C) Expression of poly-GA does not alter the degradation of IκB and phosphorylation of p65. HEK293 cells were transfected with the indicated constructs (Ct: Control; NES: NES-GA65-GFP; NLS: NLS-GA65-GFP; NT: GA65-GFP; PY: GA65-GFP-PY) and treated as described in (A). Levels of IκB and phosphorylated NF-κB p65 (P–p65) were analyzed by immunoblotting. α-tubulin served as loading control.
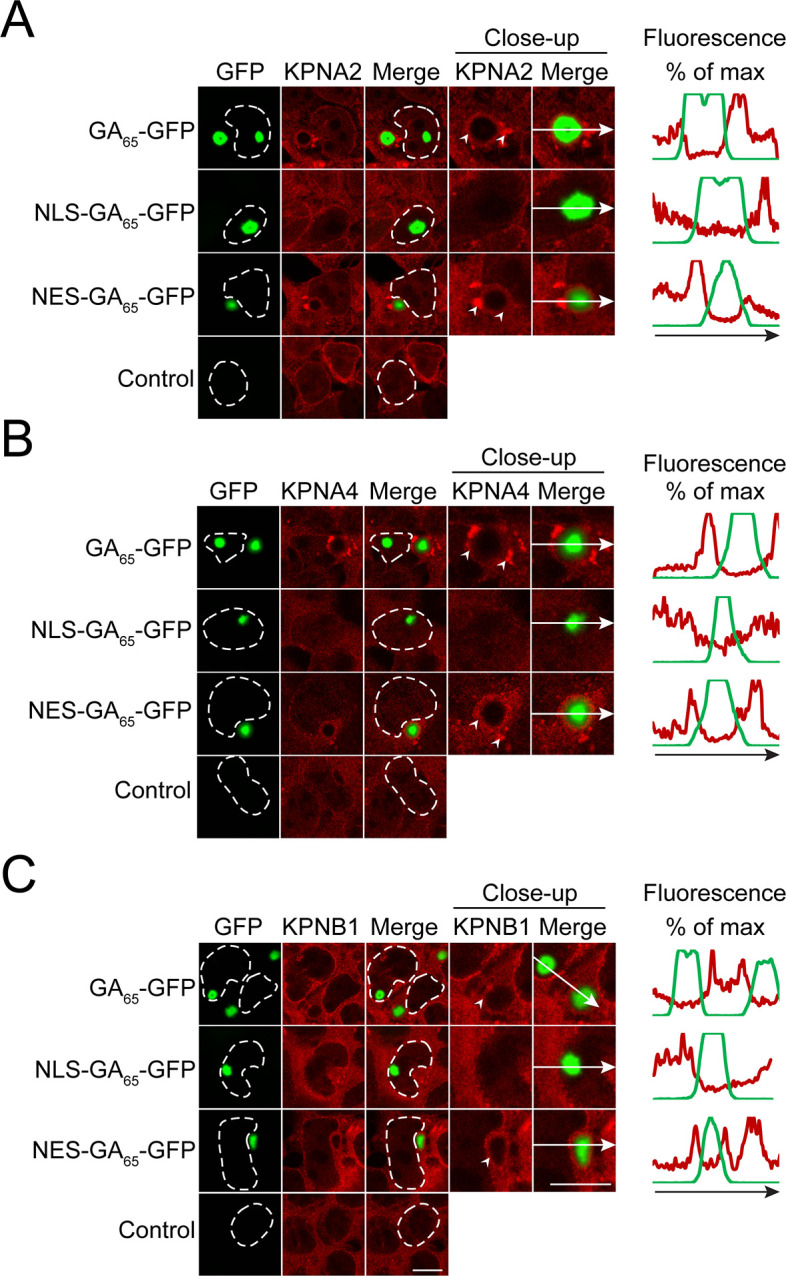
Figure 4—figure supplement 1. Importins form aggregates in cells containing cytoplasmic poly-GA aggregates and are partially sequestered.