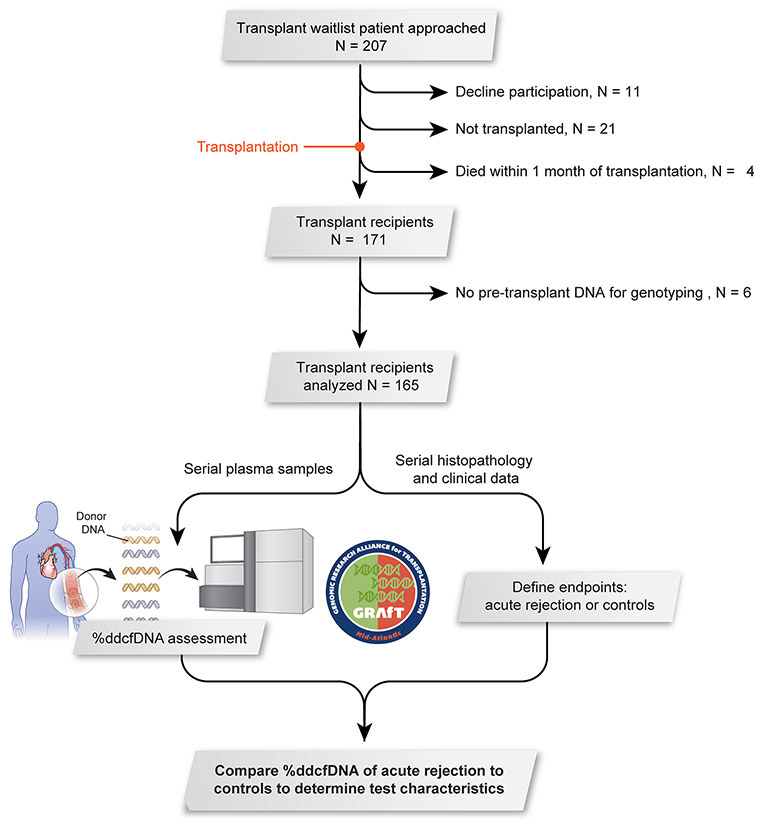
Figure 1: Study design.
Patients were recruited from five regional transplant centers. Serial plasma samples were collected at the time of routine surveillance procedures after transplant (e.g. endomyocardial biopsy or echocardiogram) and when clinically indicated monitoring was performed (e.g. graft dysfunction or suspected rejection, Table I in the Supplement). A total of 165 patients were included in this analysis and 1,867 ddcfDNA measures.