Fig. 3. GABAB–Gi coupling and G-protein selectivity.
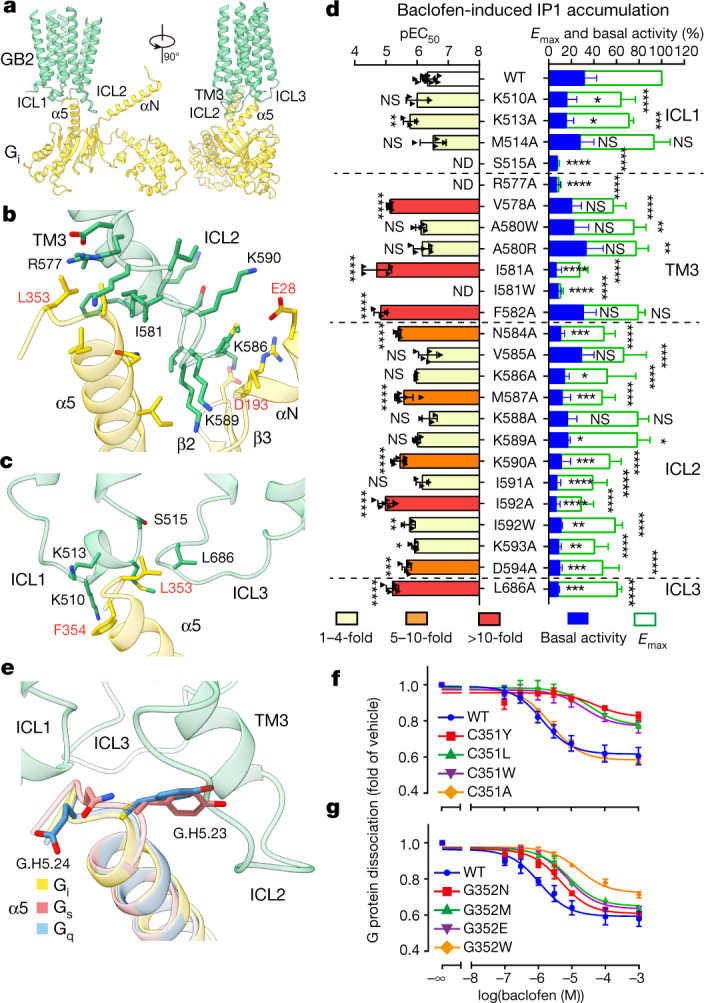
a, The Gi1 binding pocket in GABAB, which is mainly formed by three intracellular loops of GB2. GB2, green; Gαi1, yellow. b, c, Detailed interactions of the ICL2 and TM3 of GB2 with Gαi (b), and of ICL1 and ICL3 with Gαi (c). d, Baclofen-induced IP1 accumulation using Gαqi9. Bars represent differences in calculated Emax and basal activity or potency (pEC50) for each mutant as a percentage of the maximum in wild type. Data are mean ± s.e.m. from at least three independent experiments, performed in technical triplicate and analysed using one-way analysis of variance with Dunnett’s multiple comparison test to determine significance (compared with wild type). ND, not determined; NS, not significant. e, The CG.H5.23 and GG.H5.24 residues in the C-terminal α5 helix of Gαi are involved in the selective coupling between GABAB and Gi protein. The α5-helix structures of Gs (PDB 5VAI), Gq (PDB 6WHA) and the GABAB-bound Gi were aligned. f, g, Effect of CG.H5.23 (f) and GG.H5.24 (g) mutations in Gαi on GABAB–Gi coupling using NanoBiT G-protein dissociation assay. Data are mean ± s.e.m. from at least three independent experiments.
